Page 495 - The Toxicology of Fishes
P. 495
The Endocrine System 475
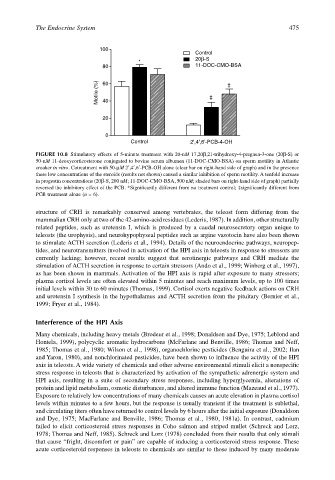
100
Control
20β-S
*
80 11-DOC-CMO-BSA
Motile (%) 60 ‡ ‡
40
20
0
Control 2',4',6'-PCB-4-OH
FIGURE 10.8 Stimulatory effects of 5-minute treatment with 20-nM 17,20β,21-trihydroxy-4-pregnen-3-one (20β-S) or
50-nM 11-deoxycorticosterone conjugated to bovine serum albumen (11-DOC-CMO-BSA) on sperm motility in Atlantic
croaker in vitro. Cotreatment with 50-µM 2′,4′,6′-PCB-OH alone (clear bar on right-hand side of graph) and in the presence
these low concentrations of the steroids (results not shown) caused a similar inhibition of sperm motility. A tenfold increase
in progestin concentrations (20β-S, 200 nM; 11-DOC-CMO-BSA, 500 nM; shaded bars on right-hand side of graph) partially
reversed the inhibitory effect of the PCB. *Significantly different from no treatment control; ‡significantly different from
PCB treatment alone (n = 6).
structure of CRH is remarkably conserved among vertebrates, the teleost form differing from the
mammalian CRH only at two of the 42-amino-acid residues (Lederis, 1987). In addition, other structurally
related peptides, such as urotensin I, which is produced by a caudal neurosecretory organ unique to
teleosts (the urophysis), and neurohypophyseal peptides such as argine vasotocin have also been shown
to stimulate ACTH secretion (Lederis et al., 1994). Details of the neuroendocrine pathways, neuropep-
tides, and neurotransmitters involved in activation of the HPI axis in teleosts in response to stressors are
currently lacking; however, recent results suggest that serotinergic pathways and CRH mediate the
stimulation of ACTH secretion in response to certain stressors (Ando et al., 1999; Winberg et al., 1997),
as has been shown in mammals. Activation of the HPI axis is rapid after exposure to many stressors;
plasma cortisol levels are often elevated within 5 minutes and reach maximum levels, up to 100 times
initial levels within 30 to 60 minutes (Thomas, 1999). Cortisol exerts negative feedback actions on CRH
and urotensin I synthesis in the hypothalamus and ACTH secretion from the pituitary (Bernier et al.,
1999; Fryer et al., 1984).
Interference of the HPI Axis
Many chemicals, including heavy metals (Brodeur et al., 1998; Donaldson and Dye, 1975; Leblond and
Hontela, 1999), polycyclic aromatic hydrocarbons (McFarlane and Benville, 1986; Thomas and Neff,
1985; Thomas et al., 1980; Wilson et al., 1998), organochlorine pesticides (Benguira et al., 2002; Ilan
and Yaron, 1980), and nonchlorinated pesticides, have been shown to influence the activity of the HPI
axis in teleosts. A wide variety of chemicals and other adverse environmental stimuli elicit a nonspecific
stress response in teleosts that is characterized by activation of the sympathetic adrenergic system and
HPI axis, resulting in a suite of secondary stress responses, including hyperglycemia, alterations of
protein and lipid metabolism, osmotic disturbances, and altered immune function (Mazeaud et al., 1977).
Exposure to relatively low concentrations of many chemicals causes an acute elevation in plasma cortisol
levels within minutes to a few hours, but the response is usually transient if the treatment is sublethal,
and circulating titers often have returned to control levels by 6 hours after the initial exposure (Donaldson
and Dye, 1975; MacFarlane and Benville, 1986; Thomas et al., 1980, 1981a). In contrast, cadmium
failed to elicit corticosteroid stress responses in Coho salmon and striped mullet (Schreck and Lorz,
1978; Thomas and Neff, 1985). Schreck and Lorz (1978) concluded from their results that only stimuli
that cause “fright, discomfort or pain” are capable of inducing a corticosteroid stress response. These
acute corticosteroid responses in teleosts to chemicals are similar to those induced by many moderate