Page 493 - The Toxicology of Fishes
P. 493
The Endocrine System 473
o,p´–DDT 120 2,2´,5´–PCB–4–OH
[ 3 H]–Estradiol Bound (%) 80 Liver ER 80 Liver ER
100
100
60
60
40
40
20
–6
–7
10
10 –7 Testis ER 10 –5 10 –4 10 –3 20 10 –8 Testis ER 10 –6 10 –5 10 –4
10
Nonylphenol 100 Zearalenone
[ 3 H]–Estradiol Bound (%) 80 80
100
60
60
40
40
20
Testis ER
Testis ER
–6
10
10 –7 Liver ER –6 10 –5 10 –4 20 10 –7 Liver ER 10 –5 10 –4 10 –3
10
Concentration (M) Concentration (M)
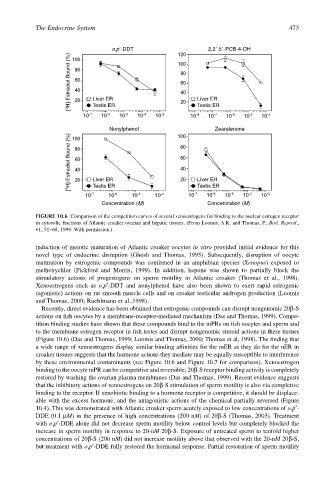
FIGURE 10.6 Comparison of the competition curves of several xenoestrogens for binding to the nuclear estrogen receptor
in cytosolic fractions of Atlantic croaker ovarian and hepatic tissues. (From Loomis, A.K. and Thomas, P., Biol. Reprod.,
61, 51–60, 1999. With permission.)
induction of meiotic maturation of Atlantic croaker oocytes in vitro provided initial evidence for this
novel type of endocrine disruption (Ghosh and Thomas, 1995). Subsequently, disruption of oocyte
maturation by estrogenic compounds was confirmed in an amphibian species (Xenopus) exposed to
methoxychlor (Pickford and Morris, 1999). In addition, kepone was shown to partially block the
stimulatory actions of progestogens on sperm motility in Atlantic croaker (Thomas et al., 1998).
Xenoestrogens such as o,p′-DDT and nonylphenol have also been shown to exert rapid estrogenic
(agonistic) actions on rat smooth muscle cells and on croaker testicular androgen production (Loomis
and Thomas, 2000; Ruehlmann et al.,1998).
Recently, direct evidence has been obtained that estrogenic compounds can disrupt nongenomic 20β-S
actions on fish oocytes by a membrane-receptor-mediated mechanism (Das and Thomas, 1999). Compe-
tition binding studies have shown that these compounds bind to the mPRs on fish oocytes and sperm and
to the membrane estrogen receptor in fish testes and disrupt nongenomic steroid actions in these tissues
(Figure 10.6) (Das and Thomas, 1999; Loomis and Thomas, 2000; Thomas et al, 1998). The finding that
a wide range of xenoestrogens display similar binding affinities for the mER as they do for the nER in
croaker tissues suggests that the hormone actions they mediate may be equally susceptible to interference
by these environmental contaminants (see Figure 10.6 and Figure 10.7 for comparison). Xenoestrogen
binding to the oocyte mPR can be competitive and reversible; 20β-S receptor binding activity is completely
restored by washing the ovarian plasma membranes (Das and Thomas, 1999). Recent evidence suggests
that the inhibitory actions of xenoestrogens on 20β-S stimulation of sperm motility is also via completive
binding to the receptor. If xenobiotic binding to a hormone receptor is competitive, it should be displace-
able with the excess hormone, and the antagonistic actions of the chemical partially reversed (Figure
10.4). This was demonstrated with Atlantic croaker sperm acutely exposed to low concentrations of o,p′-
DDE (0.1 µM) in the presence of high concentrations (200 nM) of 20β-S (Thomas, 2003). Treatment
with o,p′-DDE alone did not decrease sperm motility below control levels but completely blocked the
increase in sperm motility in response to 20-nM 20β-S. Exposure of untreated sperm to tenfold higher
concentrations of 20β-S (200 nM) did not increase motility above that observed with the 20-nM 20β-S,
but treatment with o,p′-DDE fully restored the hormonal response. Partial restoration of sperm motility