Page 565 - The Toxicology of Fishes
P. 565
Chemical Carcinogenesis in Fishes 545
CYP
O
Major Product
Benzo(a)pyrene-7,8-epoxide
Epoxide
Hydrolase
O CYP
HO HO
OH OH
Benzo(a)-pyrene-7,8-diol-9,10-epoxide Benzo(a)pyrene-7,8-diol
Carcinogen
Reacts with G
OH O
HO OH
HN N
N N N
H
DNA covalently modified
(nonreversible)
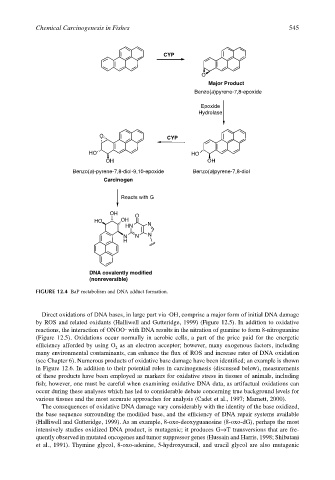
FIGURE 12.4 BaP metabolism and DNA adduct formation.
Direct oxidations of DNA bases, in large part via ·OH, comprise a major form of initial DNA damage
by ROS and related oxidants (Halliwell and Gutteridge, 1999) (Figure 12.5). In addition to oxidative
reactions, the interaction of ONOO with DNA results in the nitration of guanine to form 8-nitroguanine
–
(Figure 12.5). Oxidations occur normally in aerobic cells, a part of the price paid for the energetic
efficiency afforded by using O as an electron acceptor; however, many exogenous factors, including
2
many environmental contaminants, can enhance the flux of ROS and increase rates of DNA oxidation
(see Chapter 6). Numerous products of oxidative base damage have been identified; an example is shown
in Figure 12.6. In addition to their potential roles in carcinogenesis (discussed below), measurements
of these products have been employed as markers for oxidative stress in tissues of animals, including
fish; however, one must be careful when examining oxidative DNA data, as artifactual oxidations can
occur during these analyses which has led to considerable debate concerning true background levels for
various tissues and the most accurate approaches for analysis (Cadet et al., 1997; Marnett, 2000).
The consequences of oxidative DNA damage vary considerably with the identity of the base oxidized,
the base sequence surrounding the modified base, and the efficiency of DNA repair systems available
(Halliwell and Gutteridge, 1999). As an example, 8-oxo-deoxyguanosine (8-oxo-dG), perhaps the most
intensively studies oxidized DNA product, is mutagenic; it produces G→T transversions that are fre-
quently observed in mutated oncogenes and tumor suppressor genes (Hussain and Harris, 1998; Shibutani
et al., 1991). Thymine glycol, 8-oxo-adenine, 5-hydroxyuracil, and uracil glycol are also mutagenic