Page 113 - Feline Cardiology
P. 113
112 Section D: Cardiomyopathies
intracytosolic calcium overload, altered left ventricular
loading conditions, and myocardial ischemia from small
coronary artery disease.
• Increased ventricular stiffness is caused by concentric
left ventricular hypertrophy, myofiber disarray, and
myocardial fibrosis.
• Delayed relaxation and increased ventricular stiffness
increase LV diastolic filling pressure, which may lead to
development of left heart failure.
• Pulmonary edema and/or pleural effusion develop as
Cardiomyopathies • Systolic anterior motion (SAM) of the mitral valve
the main manifestations of left-sided congestive heart
failure in cats with HCM.
develops secondary to anterior-ventrally displaced,
hypertrophied papillary muscles that pull the mitral valve
into the left ventricular outflow tract during systole.
Other factors that may exacerbate or worsen SAM of
the mitral valve include severe basilar septal concentric
hypertrophy, increased contractility, and tachycardia.
• Moderate or severe SAM of the mitral valve greatly
increases left ventricular systolic pressure, which
increases severity of concentric LV hypertrophy and
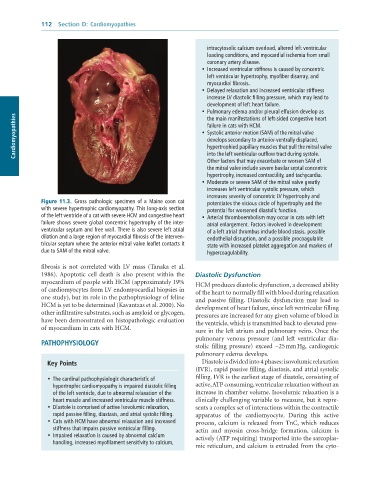
Figure 11.3. Gross pathologic specimen of a Maine coon cat potentiates the vicious circle of hypertrophy and the
with severe hypertrophic cardiomyopathy. This long-axis section potential for worsened diastolic function.
of the left ventricle of a cat with severe HCM and congestive heart • Arterial thromboembolism may occur in cats with left
failure shows severe global concentric hypertrophy of the inter- atrial enlargement. Factors involved in development
ventricular septum and free wall. There is also severe left atrial of a left atrial thrombus include blood stasis, possible
dilation and a large region of myocardial fibrosis of the interven- endothelial disruption, and a possible procoagulable
tricular septum where the anterior mitral valve leaflet contacts it state with increased platelet aggregation and markers of
due to SAM of the mitral valve. hypercoagulability.
fibrosis is not correlated with LV mass (Tanaka et al.
1986). Apoptotic cell death is also present within the Diastolic Dysfunction
myocardium of people with HCM (approximately 19% HCM produces diastolic dysfunction, a decreased ability
of cardiomyocytes from LV endomyocardial biopsies in of the heart to normally fill with blood during relaxation
one study), but its role in the pathophysiology of feline and passive filling. Diastolic dysfunction may lead to
HCM is yet to be determined (Kavantzas et al. 2000). No development of heart failure, since left ventricular filling
other infiltrative substrates, such as amyloid or glycogen, pressures are increased for any given volume of blood in
have been demonstrated on histopathologic evaluation the ventricle, which is transmitted back to elevated pres-
of myocardium in cats with HCM. sure in the left atrium and pulmonary veins. Once the
pulmonary venous pressure (and left ventricular dia-
PATHOPHYSIOLOGY stolic filling pressure) exceed ∼25 mm Hg, cardiogenic
pulmonary edema develops.
Key Points Diastole is divided into 4 phases: isovolumic relaxation
(IVR), rapid passive filling, diastasis, and atrial systolic
• The cardinal pathophysiologic characteristic of filling. IVR is the earliest stage of diastole, consisting of
hypertrophic cardiomyopathy is impaired diastolic filling active, ATP consuming, ventricular relaxation without an
of the left ventricle, due to abnormal relaxation of the increase in chamber volume. Isovolumic relaxation is a
heart muscle and increased ventricular muscle stiffness. clinically challenging variable to measure, but it repre-
• Diastole is comprised of active isovolumic relaxation, sents a complex set of interactions within the contractile
rapid passive filling, diastasis, and atrial systolic filling. apparatus of the cardiomyocyte. During this active
• Cats with HCM have abnormal relaxation and increased process, calcium is released from TnC, which reduces
stiffness that impairs passive ventricular filling. actin and myosin cross-bridge formation, calcium is
• Impaired relaxation is caused by abnormal calcium actively (ATP requiring) transported into the sarcoplas-
handling, increased myofilament sensitivity to calcium,
mic reticulum, and calcium is extruded from the cyto-