Page 115 - Feline Cardiology
P. 115
114 Section D: Cardiomyopathies
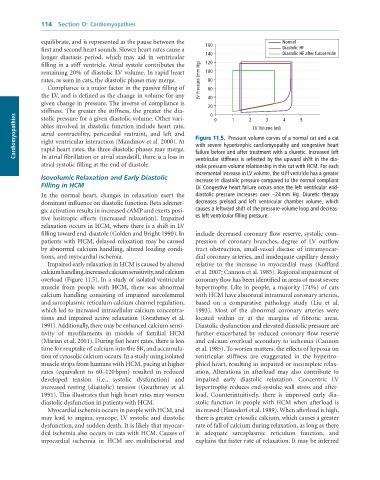
equilibrate, and is represented as the pause between the 160 Normal
first and second heart sounds. Slower heart rates cause a Diastolic HF
longer diastasis period, which may aid in ventricular 140 Diastolic HF after furosemide
filling in a stiff ventricle. Atrial systole contributes the 120
remaining 20% of diastolic LV volume. In rapid heart 100
rates, as seen in cats, the diastolic phases may merge. LV Pressure (mm Hg) 80
Compliance is a major factor in the passive filling of 60
the LV, and is defined as the change in volume for any 40
given change in pressure. The inverse of compliance is 20
stiffness. The greater the stiffness, the greater the dia- 0 0 1 2 3 4 5
Cardiomyopathies ables involved in diastolic function include heart rate, Figure 11.5. Pressure volume curves of a normal cat and a cat
stolic pressure for a given diastolic volume. Other vari-
LV Volume (ml)
atrial contractility, pericardial restraint, and left and
right ventricular interaction (Mandinov et al. 2000). At
with severe hypertrophic cardiomyopathy and congestive heart
rapid heart rates, the three diastolic phases may merge.
In atrial fibrillation or atrial standstill, there is a loss in
ventricular stiffness is reflected by the upward shift in the dia-
atrial systolic filling at the end of diastole. failure before and after treatment with a diuretic. Increased left
stolic pressure volume relationship in this cat with HCM. For each
incremental increase in LV volume, the stiff ventricle has a greater
Isovolumic Relaxation and Early Diastolic increase in diastolic pressure compared to the normal compliant
Filling in HCM LV. Congestive heart failure occurs once the left ventricular end-
In the normal heart, changes in relaxation exert the diastolic pressure increases over ∼24 mm Hg. Diuretic therapy
dominant influence on diastolic function. Beta adrener- decreases preload and left ventricular chamber volume, which
gic activation results in increased cAMP and exerts posi- causes a leftward shift of the pressure-volume loop and decreas-
tive lusitropic effects (increased relaxation). Impaired es left ventricular filling pressure.
relaxation occurs in HCM, where there is a shift in LV
filling toward end-diastole (Golden and Bright 1990). In include decreased coronary flow reserve, systolic com-
patients with HCM, delayed relaxation may be caused pression of coronary branches, degree of LV outflow
by abnormal calcium handling, altered loading condi- tract obstruction, small-vessel disease of intramyocar-
tions, and myocardial ischemia. dial coronary arteries, and inadequate capillary density
Impaired early relaxation in HCM is caused by altered relative to the increase in myocardial mass (Kofflard
calcium handling, increased calcium sensitivity, and calcium et al. 2007; Cannon et al. 1985). Regional impairment of
overload (Figure 11.5). In a study of isolated ventricular coronary flow has been identified in areas of most severe
muscle from people with HCM, there was abnormal hypertrophy. Like in people, a majority (74%) of cats
calcium handling consisting of impaired sarcolemmal with HCM have abnormal intramural coronary arteries,
and sarcoplasmic reticulum calcium channel regulation, based on a comparative pathology study (Liu et al.
which led to increased intracellular calcium concentra- 1993). Most of the abnormal coronary arteries were
tions and impaired active relaxation (Gwathmey et al. located within or at the margins of fibrotic areas.
1991). Additionally, there may be enhanced calcium sensi- Diastolic dysfunction and elevated diastolic pressure are
tivity of myofilaments in models of familial HCM further exacerbated by reduced coronary flow reserve
(Marian et al. 2001). During fast heart rates, there is less and calcium overload secondary to ischemia (Cannon
time for reuptake of calcium into the SR, and accumula- et al. 1985). To worsen matters, the effects of hypoxia on
tion of cytosolic calcium occurs. In a study using isolated ventricular stiffness are exaggerated in the hypertro-
muscle strips from humans with HCM, pacing at higher phied heart, resulting in impaired or incomplete relax-
rates (equivalent to 60–120 bpm) resulted in reduced ation. Alterations in afterload may also contribute to
developed tension (i.e., systolic dysfunction) and impaired early diastolic relaxation. Concentric LV
increased resting (diastolic) tension (Gwathmey et al. hypertrophy reduces end-systolic wall stress and after-
1991). This illustrates that high heart rates may worsen load. Counterintuitively, there is improved early dia-
diastolic dysfunction in patients with HCM. stolic function in people with HCM when afterload is
Myocardial ischemia occurs in people with HCM, and increased (Hausdorf et al. 1989). When afterload is high,
may lead to angina, syncope, LV systolic and diastolic there is greater cytosolic calcium, which causes a greater
dysfunction, and sudden death. It is likely that myocar- rate of fall of calcium during relaxation, as long as there
dial ischemia also occurs in cats with HCM. Causes of is adequate sarcoplasmic reticulum function, and
myocardial ischemia in HCM are multifactorial and explains the faster rate of relaxation. It may be inferred