Page 711 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 711
676 SECTION | IX Gases, Solvents and Other Industrial Toxicants
VetBooks.ir pigments in carbonless copy paper (Headrick et al.,
1999; Loganathan and Lam, 2012). The PCB products
in the United States had the trade name Aroclor fol-
lowed by four digits that identified the particular mix-
ture. The first two digits referred to the 12 carbon
atoms of the biphenyl molecule, and the last two digits
referred to the percent of chlorine in the mixture, by
weight (i.e., Aroclor 1254 has 54% chlorine by
weight). Similar commercial PCB mixtures were pro-
duced by other manufacturers worldwide, including the
Clophens (Germany), Phenoclors and Pyralenes
(France), Fenclors (Italy), and Kanechlors (Japan)
(Kimbrough, 1995; Safe, 1994; Kodavanti et al.,
2014a,b).
The physical and chemical properties of PCBs, such
as high stability, inertness, and dielectric properties,
which were advantageous for many industrial purposes,
led to the indiscriminate use of PCBs in large quantities
(Loganathan and Lam, 2012; Kodavanti et al., 2014a,b).
For example, the estimated cumulative production of
PCBs in the United States between 1930 and 1975 was
700,000 tons. About 1.2 million tons were estimated to
have been produced worldwide (Kimbrough, 1995;
Tanabe, 1988). As a result of widespread use, PCBs
were identified in environmental media and biota. Due
to their widespread environmental contamination in
the 1970s, PCB production decreased, and eventually,
ceased (Loganathan and Kannan, 1994). Nevertheless,
PCBs are still found in the environment, as well as in
biological tissues due to their persistence. About 31%
(370,000 tons) of all the PCBs produced is present in
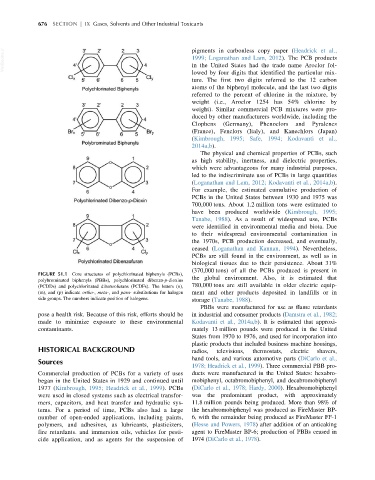
FIGURE 51.1 Core structures of polychlorinated biphenyls (PCBs),
polybrominated biphenyls (PBBs), polychlorinated dibenzo-p-dioxins the global environment. Also, it is estimated that
(PCDDs) and polychlorinated dibenzofurans (PCDFs). The letters (o), 780,000 tons are still available in older electric equip-
(m), and (p) indicate ortho-, meta-, and para- substitutions for halogen ment and other products deposited in landfills or in
side groups. The numbers indicate position of halogens. storage (Tanabe, 1988).
PBBs were manufactured for use as flame retardants
poseahealthrisk. Becauseofthis risk, efforts should be in industrial and consumer products (Damstra et al., 1982;
made to minimize exposure to these environmental Kodavanti et al., 2014a,b). It is estimated that approxi-
contaminants. mately 13 million pounds were produced in the United
States from 1970 to 1976, and used for incorporation into
plastic products that included business machine housings,
HISTORICAL BACKGROUND radios, televisions, thermostats, electric shavers,
hand tools, and various automotive parts (DiCarlo et al.,
Sources
1978; Headrick et al., 1999). Three commercial PBB pro-
Commercial production of PCBs for a variety of uses ducts were manufactured in the United States: hexabro-
beganinthe UnitedStatesin1929andcontinueduntil mobiphenyl, octabromobiphenyl, and decabromobiphenyl
1977 (Kimbrough, 1995; Headrick et al., 1999). PCBs (DiCarlo et al., 1978; Hardy, 2000). Hexabromobiphenyl
were used in closed systems such as electrical transfor- was the predominant product, with approximately
mers, capacitors, and heat transfer and hydraulic sys- 11.8 million pounds being produced. More than 98% of
tems. For a period of time, PCBs also had a large the hexabromobiphenyl was produced as FireMaster BP-
number of open-ended applications, including paints, 6, with the remainder being produced as FireMaster FF-1
polymers, and adhesives, as lubricants, plasticizers, (Hesse and Powers, 1978) after addition of an anticaking
fire retardants. and immersion oils, vehicles for pesti- agent to FireMaster BP-6; production of PBBs ceased in
cide application, and as agents for the suspension of 1974 (DiCarlo et al., 1978).