Page 736 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 736
Brominated Flame Retardants and Perfluorinated Chemicals Chapter | 52 701
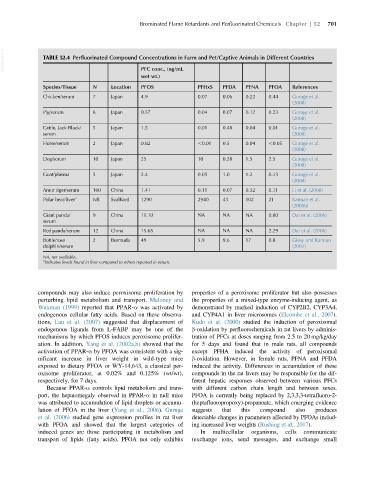
VetBooks.ir TABLE 52.4 Perfluorinated Compound Concentrations in Farm and Pet/Captive Animals in Different Countries
PFC conc., (ng/mL
wet wt.)
Species/Tissue N Location PFOS PFHxS PFDA PFNA PFOA References
Chicken/serum 7 Japan 4.9 0.07 0.06 0.22 0.44 Guruge et al.
(2008)
Pig/serum 6 Japan 0.57 0.04 0.07 0.12 0.23 Guruge et al.
(2008)
Cattle, Jack Black/ 5 Japan 1.5 0.01 0.48 0.04 0.01 Guruge et al.
serum (2008)
Horse/serum 2 Japan 0.82 ,0.01 0.5 0.04 ,0.05 Guruge et al.
(2008)
Dog/serum 10 Japan 25 10 0.28 1.5 2.5 Guruge et al.
(2008)
Goat/plasma 5 Japan 2.4 0.05 1.0 1.2 0.13 Guruge et al.
(2008)
Amur tiger/serum 100 China 1.41 0.11 0.07 0.32 0.11 Li et al. (2008)
Polar bear/liver a NR Svalbard 1290 2940 43 102 21 Kannan et al.
(2005b)
Giant panda/ 9 China 11.10 NA NA NA 0.80 Dai et al. (2006)
serum
Red panda/serum 12 China 15.65 NA NA NA 2.29 Dai et al. (2006)
Bottlenose 2 Bermuda 49 5.9 9.6 17 0.8 Giesy and Kannan
dolphin/serum (2001)
NA, not available.
a Indicates levels found in liver compared to others reported in serum.
compounds may also induce peroxisome proliferation by properties of a peroxisome proliferator but also possesses
perturbing lipid metabolism and transport. Maloney and the properties of a mixed-type enzyme-inducing agent, as
Waxman (1999) reported that PPAR-α was activated by demonstrated by marked induction of CYP2B2, CYP3A4,
endogenous cellular fatty acids. Based on these observa- and CYP4A1 in liver microsomes (Elcombe et al., 2007).
tions, Lau et al. (2007) suggested that displacement of Kudo et al. (2000) studied the induction of peroxisomal
endogenous ligands from L-FABP may be one of the β-oxidation by perfluorochemicals in rat livers by adminis-
mechanisms by which PFOS induces peroxisome prolifer- tration of PFCs at doses ranging from 2.5 to 20 mg/kg/day
ation. In addition, Yang et al. (2002a,b) showed that the for 5 days and found that in male rats, all compounds
activation of PPAR-α by PFOA was consistent with a sig- except PFHA induced the activity of peroxisomal
nificant increase in liver weight in wild-type mice β-oxidation. However, in female rats, PFNA and PFDA
exposed to dietary PFOA or WY-14,643, a classical per- induced the activity. Differences in accumulation of these
oxisome proliferator, at 0.02% and 0.125% (wt/wt), compounds in the rat livers may be responsible for the dif-
respectively, for 7 days. ferent hepatic responses observed between various PFCs
Because PPAR-α controls lipid metabolism and trans- with different carbon chain length and between sexes.
port, the hepatomegaly observed in PPAR-α in null mice PFOA is currently being replaced by 2,3,3,3-tetrafluoro-2-
was attributed to accumulation of lipid droplets or accumu- (heptafluoropropoxy)-propanoate, which emerging evidence
lation of PFOA in the liver (Yang et al., 2006). Guruge suggests that this compound also produces
et al. (2006) studied gene expression profiles in rat liver detectable changes in parameters affected by PFOAs includ-
with PFOA and showed that the largest categories of ing increased liver weights (Rushing et al., 2017).
induced genes are those participating in metabolism and In multicellular organisms, cells communicate
transport of lipids (fatty acids). PFOA not only exhibits (exchange ions, send messages, and exchange small