Page 734 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 734
Brominated Flame Retardants and Perfluorinated Chemicals Chapter | 52 699
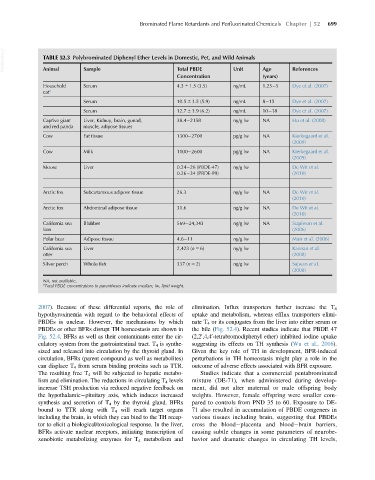
VetBooks.ir TABLE 52.3 Polybrominated Diphenyl Ether Levels in Domestic, Pet, and Wild Animals
Total PBDE
Sample
Animal
(years)
Concentration Unit Age References
Household Serum 4.3 6 1.5 (3.5) ng/mL 1.25 5 Dye et al. (2007)
cat a
Serum 10.5 6 3.5 (5.9) ng/mL 8 15 Dye et al. (2007)
Serum 12.7 6 3.9 (6.2) ng/mL 10 18 Dye et al. (2007)
Captive giant Liver, Kidney, brain, gonad, 38.4 2158 ng/g lw NA Hu et al. (2008)
and red panda muscle, adipose tissues
Cow Fat tissue 1300 2700 pg/g lw NA Kierkegaard et al.
(2009)
Cow Milk 1100 2600 pg/g lw NA Kierkegaard et al.
(2009)
Moose Liver 0.24 26 (PBDE-47) ng/g lw De Wit et al.
0.26 34 (PBDE-99) (2010)
Arctic fox Subcutaneous adipose tissue 26.3 ng/g lw NA De Wit et al.
(2010)
Arctic fox Abdominal adipose tissue 31.6 ng/g lw NA De Wit et al.
(2010)
California sea Blubber 569 24,343 ng/g lw NA Stapleton et al.
lion (2006)
Polar bear Adipose tissue 4.6 11 ng/g lw Muir et al. (2006)
California sea Liver 2,423 (n 5 6) ng/g lw Kannan et al.
otter (2008)
Silver perch Whole fish 337 (n 5 2) ng/g lw Sajwan et al.
(2008)
NA, not available.
a
Total PBDE concentrations in parentheses indicate median; lw, lipid weight.
2007). Because of these differential reports, the role of elimination. Influx transporters further increase the T 4
hypothyroxinemia with regard to the behavioral effects of uptake and metabolism, whereas efflux transporters elimi-
PBDEs is unclear. However, the mechanisms by which nate T 4 or its conjugates from the liver into either serum or
PBDEs or other BFRs disrupt TH homeostasis are shown in thebile(Fig. 52.4). Recent studies indicate that PBDE 47
Fig. 52.4. BFRs as well as their contaminants enter the cir- (2,2 ,4,4 -tetrabromodiphenyl ether) inhibited iodine uptake
0
0
culatory system from the gastrointestinal tract. T 4 is synthe- suggesting its effects on TH synthesis (Wu et al., 2016).
sized and released into circulation by the thyroid gland. In Given the key role of TH in development, BFR-induced
circulation, BFRs (parent compound as well as metabolites) perturbations in TH homeostasis might play a role in the
can displace T 4 from serum binding proteins such as TTR. outcome of adverse effects associated with BFR exposure.
The resulting free T 4 will be subjected to hepatic metabo- Studies indicate that a commercial pentabrominated
lism and elimination. The reductions in circulating T 4 levels mixture (DE-71), when administered during develop-
increase TSH production via reduced negative feedback on ment, did not alter maternal or male offspring body
the hypothalamic pituitary axis, which induces increased weights. However, female offspring were smaller com-
synthesis and secretion of T 4 by the thyroid gland. BFRs pared to controls from PND 35 to 60. Exposure to DE-
bound toTTR alongwithT 4 will reach target organs 71 also resulted in accumulation of PBDE congeners in
including the brain, in which they can bind to the TH recep- various tissues including brain, suggesting that PBDEs
tor to elicit a biological/toxicological response. In the liver, cross the blood placenta and blood brain barriers,
BFRs activate nuclear receptors, initiating transcription of causing subtle changes in some parameters of neurobe-
xenobiotic metabolizing enzymes for T 4 metabolism and havior and dramatic changes in circulating TH levels,