Page 722 - Small Animal Internal Medicine, 6th Edition
P. 722
694 PART V Urinary Tract Disorders
30 of renal disease. Tubular cells have receptors for hormones
GFR and growth factors, some of which are small-MW proteins
VetBooks.ir 20 (20) tubular cells, where they promote cellular proliferation and
~50%
that are filtered excessively and taken up by the proximal
extracellular matrix deposition, leading to tubulointerstitial
10
damage. Tubular cell overload caused by increased reabsorp-
0 tion of filtered proteins also upregulates inflammatory and
0 0 10 20 30 40 50 60 70 80 vasoactive genes that contribute to damage. Ischemia of por-
tions of the nephron downstream from the damaged glomer-
30 ulus, mineralization in the kidney, and local ammonia
(37.5) 100% accumulation are additional factors that contribute to tubu-
GFR
Percent of total nephrons 10 0 0 0 10 20 30 40 50 60 70 80 of the activity of the underlying primary renal disease.
20
lointerstitial lesions. This progressive damage is independent
Factors that may affect the progression of CKD include
species differences, extent and duration of reduction in renal
mass, dietary modifications, and complications. In dogs and
in progression, whereas progression occurs in humans and
40 (37.5) cats, 85% to 95% of renal tissue must be destroyed to result
rats after 75% to 80% renal ablation. Dogs with 75% reduc-
tion in renal mass followed for 4 years did not show evidence
30
of progression, whereas dogs with 94% reduction in renal
mass developed progression over 24 months. In rats, dietary
20
restriction of protein can reverse glomerular hyperfiltration.
In one study of dogs, however, a diet containing 17% protein
10
did not prevent glomerular hyperfiltration in dogs with 94%
0 renal ablation. On the other hand, a diet containing 8%
0 0 10 20 30 40 50 60 70 80 protein has been shown to result in malnutrition (e.g., weight
SNGFR (nL/min) loss, hypoalbuminemia) and increased mortality in dogs
with experimentally induced renal disease. Decreasing
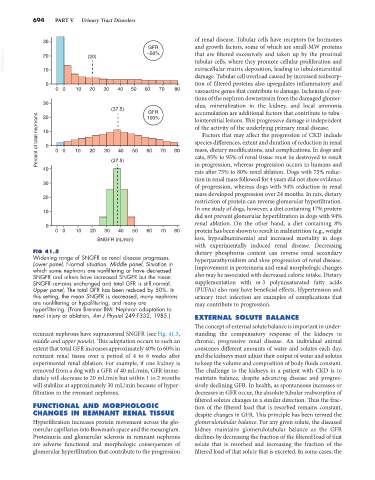
FIG 41.5 dietary phosphorus content can reverse renal secondary
Widening range of SNGFR as renal disease progresses. hyperparathyroidism and slow progression of renal disease.
Lower panel, Normal situation. Middle panel, Situation in
which some nephrons are nonfiltering or have decreased Improvement in proteinuria and renal morphologic changes
SNGFR and others have increased SNGFR but the mean also may be associated with decreased caloric intake. Dietary
SNGFR remains unchanged and total GFR is still normal. supplementation with ω-3 polyunsaturated fatty acids
Upper panel, The total GFR has been reduced by 50%. In (PUFAs) also may have beneficial effects. Hypertension and
this setting, the mean SNGFR is decreased, many nephrons urinary tract infection are examples of complications that
are nonfiltering or hypofiltering, and many are may contribute to progression.
hyperfiltering. (From Brenner BM: Nephron adaptation to
renal injury or ablation, Am J Physiol 249:F332, 1985.) EXTERNAL SOLUTE BALANCE
The concept of external solute balance is important in under-
remnant nephrons have supranormal SNGFR (see Fig. 41.5, standing the compensatory response of the kidneys to
middle and upper panels). This adaptation occurs to such an chronic, progressive renal disease. An individual animal
extent that total GFR increases approximately 40% to 60% in consumes different amounts of water and solutes each day,
remnant renal tissue over a period of 4 to 6 weeks after and the kidneys must adjust their output of water and solutes
experimental renal ablation. For example, if one kidney is to keep the volume and composition of body fluids constant.
removed from a dog with a GFR of 40 mL/min, GFR imme- The challenge to the kidneys in a patient with CKD is to
diately will decrease to 20 mL/min but within 1 to 2 months maintain balance, despite advancing disease and progres-
will stabilize at approximately 30 mL/min because of hyper- sively declining GFR. In health, as spontaneous increases or
filtration in the remnant nephrons. decreases in GFR occur, the absolute tubular reabsorption of
filtered solutes changes in a similar direction. Thus the frac-
FUNCTIONAL AND MORPHOLOGIC tion of the filtered load that is resorbed remains constant,
CHANGES IN REMNANT RENAL TISSUE despite changes in GFR. This principle has been termed the
Hyperfiltration increases protein movement across the glo- glomerulotubular balance. For any given solute, the diseased
merular capillaries into Bowman’s space and the mesangium. kidney maintains glomerulotubular balance as the GFR
Proteinuria and glomerular sclerosis in remnant nephrons declines by decreasing the fraction of the filtered load of that
are adverse functional and morphologic consequences of solute that is resorbed and increasing the fraction of the
glomerular hyperfiltration that contribute to the progression filtered load of that solute that is excreted. In some cases, the