Page 280 - Withrow and MacEwen's Small Animal Clinical Oncology, 6th Edition
P. 280
CHAPTER 15 Molecular/Targeted Therapy of Cancer 259
Growth
factor
VetBooks.ir Receptor
P P SOS RAS GDP RAS GTP
P P SHC GRB2
RAF P
P
P
MEK
P P
ERK
P
p90RSK ELK1
Nucleus
Gene transcription
cell cycle progression
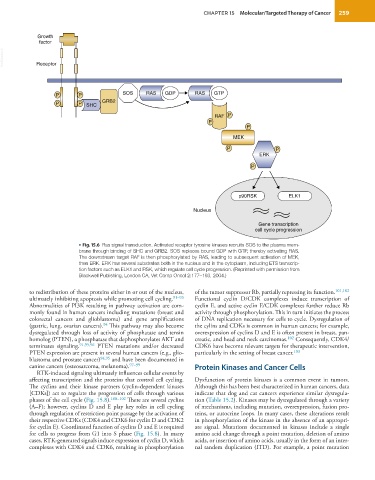
• Fig. 15.6 Ras signal transduction. Activated receptor tyrosine kinases recruits SOS to the plasma mem-
brane through binding of SHC and GRB2. SOS replaces bound GDP with GTP, thereby activating RAS.
The downstream target RAF is then phosphorylated by RAS, leading to subsequent activation of MEK,
then ERK. ERK has several substrates both in the nucleus and in the cytoplasm, including ETS transcrip-
tion factors such as ELK1 and RSK, which regulate cell cycle progression. (Reprinted with permission from
Blackwell Publishing, London CA, Vet Comp Oncol 2:177–193, 2004.)
to redistribution of these proteins either in or out of the nucleus, of the tumor suppressor Rb, partially repressing its function. 101,102
ultimately inhibiting apoptosis while promoting cell cycling. 91–93 Functional cyclin D/CDK complexes induce transcription of
Abnormalities of PI3K resulting in pathway activation are com- cyclin E, and active cyclin E/CDK complexes further reduce Rb
monly found in human cancers including mutations (breast and activity through phosphorylation. This in turn initiates the process
colorectal cancers and glioblastoma) and gene amplifications of DNA replication necessary for cells to cycle. Dysregulation of
94
(gastric, lung, ovarian cancers). This pathway may also become the cylins and CDKs is common in human cancers; for example,
dysregulated through loss of activity of phosphatase and tensin overexpression of cyclins D and E is often present in breast, pan-
homolog (PTEN), a phosphatase that dephosphorylates AKT and creatic, and head and neck carcinomas. 102 Consequently, CDK4/
terminates signaling. 91,95,96 PTEN mutations and/or decreased CDK6 have become relevant targets for therapeutic intervention,
PTEN expression are present in several human cancers (e.g., glio- particularly in the setting of breast cancer. 103
blastoma and prostate cancer) 94,95 and have been documented in
canine cancers (osteosarcoma, melanoma). 97–99 Protein Kinases and Cancer Cells
RTK-induced signaling ultimately influences cellular events by
affecting transcription and the proteins that control cell cycling. Dysfunction of protein kinases is a common event in tumors.
The cyclins and their kinase partners (cyclin-dependent kinases Although this has been best characterized in human cancers, data
[CDKs]) act to regulate the progression of cells through various indicate that dog and cat cancers experience similar dysregula-
phases of the cell cycle (Fig. 15.8). 100–102 There are several cyclins tion (Table 15.2). Kinases may be dysregulated through a variety
(A–F); however, cyclins D and E play key roles in cell cycling of mechanisms, including mutation, overexpression, fusion pro-
through regulation of restriction point passage by the activation of teins, or autocrine loops. In many cases, these alterations result
their respective CDKs (CDK4 and CDK6 for cyclin D and CDK2 in phosphorylation of the kinase in the absence of an appropri-
for cyclin E). Coordinated function of cyclins D and E is required ate signal. Mutations documented in kinases include a single
for cells to progress from G1 into S phase (Fig. 15.8). In many amino acid change through a point mutation, deletion of amino
cases, RTK-generated signals induce expression of cyclin D, which acids, or insertion of amino acids, usually in the form of an inter-
complexes with CDK4 and CDK6, resulting in phosphorylation nal tandem duplication (ITD). For example, a point mutation