Page 58 - Withrow and MacEwen's Small Animal Clinical Oncology, 6th Edition
P. 58
CHAPTER 2 Tumor Biology and Metastasis 37
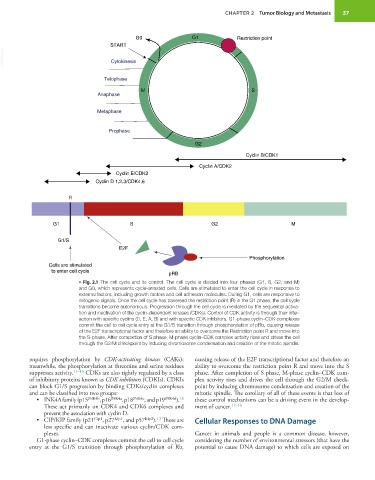
G0 G1 Restriction point
START
VetBooks.ir Cytokinesis
Telophase
M S
Anaphase
Metaphase
Prophase
G2
Cyclin B/CDK1
Cyclin A/CDK2
Cyclin E/CDK2
Cyclin D 1,2,3/CDK4,6
R
G1 S G2 M
G1/S
E2F
Phosphorylation
Cells are stimulated
to enter cell cycle
pRB
• Fig. 2.1 The cell cycle and its control. The cell cycle is divided into four phases (G1, S, G2, and M)
and G0, which represents cycle-arrested cells. Cells are stimulated to enter the cell cycle in response to
external factors, including growth factors and cell adhesion molecules. During G1, cells are responsive to
mitogenic signals. Once the cell cycle has traversed the restriction point (R) in the G1 phase, the cell cycle
transitions become autonomous. Progression through the cell cycle is mediated by the sequential activa-
tion and inactivation of the cyclin-dependent kinases (CDKs). Control of CDK activity is through their inter-
action with specific cyclins (D, E, A, B) and with specific CDK inhibitors. G1-phase cyclin–CDK complexes
commit the cell to cell cycle entry at the G1/S transition through phosphorylation of pRb, causing release
of the E2F transcriptional factor and therefore an ability to overcome the Restriction point R and move into
the S-phase. After completion of S phase, M-phase cyclin–CDK complex activity rises and drives the cell
through the G2/M checkpoint by inducing chromosome condensation and creation of the mitotic spindle.
requires phosphorylation by CDK-activating kinases (CAKs); causing release of the E2F transcriptional factor and therefore an
meanwhile, the phosphorylation at threonine and serine residues ability to overcome the restriction point R and move into the S
suppresses activity. 11–16 CDKs are also tightly regulated by a class phase. After completion of S phase, M-phase cyclin–CDK com-
of inhibitory proteins known as CDK inhibitors (CDKIs). CDKIs plex activity rises and drives the cell through the G2/M check-
can block G1/S progression by binding CDKs/cyclin complexes point by inducing chromosome condensation and creation of the
and can be classified into two groups: mitotic spindle. The corollary of all of these events is that loss of
• INK4A family (p15 INK4b , p16 INK4a , p18 INK4c , and p19 INK4d 13 these control mechanisms can be a driving event in the develop-
).
These act primarily on CDK4 and CDK6 complexes and ment of cancer. 11–16
prevent the association with cyclin D.
• CIP/KIP family (p21 Cip1 , p27 Kip1 , and p57 Kip2 13 Cellular Responses to DNA Damage
). These are
less specific and can inactivate various cyclin/CDK com-
plexes. Cancer in animals and people is a common disease; however,
G1-phase cyclin–CDK complexes commit the cell to cell cycle considering the number of environmental stressors (that have the
entry at the G1/S transition through phosphorylation of Rb, potential to cause DNA damage) to which cells are exposed on