Page 59 - Withrow and MacEwen's Small Animal Clinical Oncology, 6th Edition
P. 59
38 PART I The Biology and Pathogenesis of Cancer
Active Cyclin-CDK
VetBooks.ir
DNA
Repair Apoptosis P Cdc25 Cell Cycle Apoptosis
Proteins Proteins Inhibitors Proteins
P P
E2F-1
p53
ATR ATM
p53
DNA Damage
MDM2
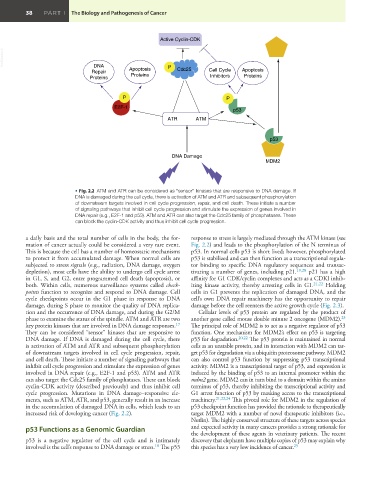
• Fig. 2.2 ATM and ATR can be considered as “sensor” kinases that are responsive to DNA damage. If
DNA is damaged during the cell cycle, there is activation of ATM and ATR and subsequent phosphorylation
of downstream targets involved in cell cycle progression, repair, and cell death. These initiate a number
of signaling pathways that inhibit cell cycle progression and stimulate the expression of genes involved in
DNA repair (e.g., E2F-1 and p53). ATM and ATR can also target the Cdc25 family of phosphatases. These
can block the cyclin-CDK activity and thus inhibit cell cycle progression.
a daily basis and the total number of cells in the body, the for- response to stress is largely mediated through the ATM kinase (see
mation of cancer actually could be considered a very rare event. Fig. 2.2) and leads to the phosphorylation of the N terminus of
This is because the cell has a number of homeostatic mechanisms p53. In normal cells p53 is short lived; however, phosphorylated
to protect it from accumulated damage. When normal cells are p53 is stabilized and can then function as a transcriptional regula-
subjected to stress signals (e.g., radiation, DNA damage, oxygen tor binding to specific DNA regulatory sequences and transac-
depletion), most cells have the ability to undergo cell cycle arrest tivating a number of genes, including p21. 19,20 p21 has a high
in G1, S, and G2, enter programmed cell death (apoptosis), or affinity for G1 CDK/cyclin complexes and acts as a CDKI inhib-
both. Within cells, numerous surveillance systems called check- iting kinase activity, thereby arresting cells in G1. 21,22 Holding
points function to recognize and respond to DNA damage. Cell cells in G1 prevents the replication of damaged DNA, and the
cycle checkpoints occur in the G1 phase in response to DNA cell’s own DNA repair machinery has the opportunity to repair
damage, during S phase to monitor the quality of DNA replica- damage before the cell reenters the active growth cycle (Fig. 2.3).
tion and the occurrence of DNA damage, and during the G2/M Cellular levels of p53 protein are regulated by the product of
phase to examine the status of the spindle. ATM and ATR are two another gene called mouse double minute 2 oncogene (MDM2).
23
key protein kinases that are involved in DNA damage responses. The principal role of MDM2 is to act as a negative regulator of p53
17
They can be considered “sensor” kinases that are responsive to function. One mechanism for MDM2’s effect on p53 is targeting
DNA damage. If DNA is damaged during the cell cycle, there p53 for degradation. 20,22 The p53 protein is maintained in normal
is activation of ATM and ATR and subsequent phosphorylation cells as an unstable protein, and its interaction with MDM2 can tar-
of downstream targets involved in cell cycle progression, repair, get p53 for degradation via a ubiquitin proteosome pathway. MDM2
and cell death. These initiate a number of signaling pathways that can also control p53 function by suppressing p53 transcriptional
inhibit cell cycle progression and stimulate the expression of genes activity. MDM2 is a transcriptional target of p53, and expression is
involved in DNA repair (e.g., E2F-1 and p53). ATM and ATR induced by the binding of p53 to an internal promoter within the
can also target the Cdc25 family of phosphatases. These can block mdm2 gene. MDM2 can in turn bind to a domain within the amino
cyclin-CDK activity (described previously) and thus inhibit cell terminus of p53, thereby inhibiting the transcriptional activity and
cycle progression. Mutations in DNA damage–responsive ele- G1 arrest function of p53 by masking access to the transcriptional
ments, such as ATM, ATR, and p53, generally result in an increase machinery. 21,22,24 This pivotal role for MDM2 in the regulation of
in the accumulation of damaged DNA in cells, which leads to an p53 checkpoint function has provided the rationale to therapeutically
increased risk of developing cancer (Fig. 2.2). target MDM2 with a number of novel therapeutic inhibitors (i.e.,
Nutlin). The highly conserved structure of these targets across species
p53 Functions as a Genomic Guardian and expected activity in many cancers provides a strong rationale for
the development of these agents in veterinary patients. The recent
p53 is a negative regulator of the cell cycle and is intimately discovery that elephants have multiple copies of p53 may explain why
18
25
involved is the cell’s response to DNA damage or stress. The p53 this species has a very low incidence of cancer.