Page 68 - Withrow and MacEwen's Small Animal Clinical Oncology, 6th Edition
P. 68
CHAPTER 2 Tumor Biology and Metastasis 47
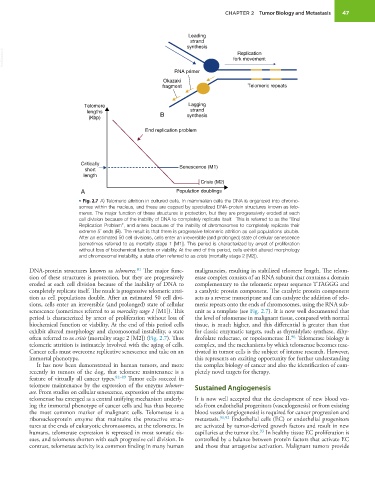
Leading
strand
synthesis
VetBooks.ir fork movement
Replication
RNA primer
Okazaki
fragment Telomeric repeats
Telomere Lagging
lengths B strand
(Kbp) synthesis
End replication problem
Critically Senescence (M1)
short
length
Crisis (M2)
A Population doublings
• Fig. 2.7 A) Telomeric attrition in cultured cells. In mammalian cells the DNA is organized into chromo-
somes within the nucleus, and these are capped by specialized DNA-protein structures known as telo-
meres. The major function of these structures is protection, but they are progressively eroded at each
cell division because of the inability of DNA to completely replicate itself. This is referred to as the “End
Replication Problem”, and arises because of the inability of chromosomes to completely replicate their
extreme 5 ends (B). The result is that there is progressive telomeric attrition as cell populations double.
′
After an estimated 50 cell divisions, cells enter an irreversible (and prolonged) state of cellular senescence
(sometimes referred to as mortality stage 1 [M1]). This period is characterized by arrest of proliferation
without loss of biochemical function or viability. At the end of this period, cells exhibit altered morphology
and chromosomal instability, a state often referred to as crisis (mortality stage 2 [M2]).
DNA-protein structures known as telomeres. The major func- malignancies, resulting in stabilized telomere length. The telom-
81
tion of these structures is protection, but they are progressively erase complex consists of an RNA subunit that contains a domain
eroded at each cell division because of the inability of DNA to complementary to the telomeric repeat sequence TTAGGG and
completely replicate itself. The result is progressive telomeric attri- a catalytic protein component. The catalytic protein component
tion as cell populations double. After an estimated 50 cell divi- acts as a reverse transcriptase and can catalyze the addition of telo-
sions, cells enter an irreversible (and prolonged) state of cellular meric repeats onto the ends of chromosomes, using the RNA sub-
senescence (sometimes referred to as mortality stage 1 [M1]). This unit as a template (see Fig. 2.7). It is now well documented that
period is characterized by arrest of proliferation without loss of the level of telomerase in malignant tissue, compared with normal
biochemical function or viability. At the end of this period cells tissue, is much higher, and this differential is greater than that
exhibit altered morphology and chromosomal instability, a state for classic enzymatic targets, such as thymidylate synthase, dihy-
often referred to as crisis (mortality stage 2 [M2]) (Fig. 2.7). Thus drofolate reductase, or topoisomerase II. Telomerase biology is
90.
telomeric attrition is intimately involved with the aging of cells. complex, and the mechanisms by which telomerase becomes reac-
Cancer cells must overcome replicative senescence and take on an tivated in tumor cells is the subject of intense research. However,
immortal phenotype. this represents an exciting opportunity for further understanding
It has now been demonstrated in human tumors, and more the complex biology of cancer and also the identification of com-
recently in tumors of the dog, that telomere maintenance is a pletely novel targets for therapy.
feature of virtually all cancer types. 82–89 Tumor cells succeed in
telomere maintenance by the expression of the enzyme telomer- Sustained Angiogenesis
ase. From studies on cellular senescence, expression of the enzyme
telomerase has emerged as a central unifying mechanism underly- It is now well accepted that the development of new blood ves-
ing the immortal phenotype of cancer cells and has thus become sels from endothelial progenitors (vasculogenesis) or from existing
the most common marker of malignant cells. Telomerase is a blood vessels (angiogenesis) is required for cancer progression and
ribonucleoprotein enzyme that maintains the protective struc- metastasis. 91,92 Endothelial cells (EC) or endothelial progenitors
tures at the ends of eukaryotic chromosomes, at the telomeres. In are activated by tumor-derived growth factors and result in new
93
humans, telomerase expression is repressed in most somatic tis- capillaries at the tumor site. In healthy tissue EC proliferation is
sues, and telomeres shorten with each progressive cell division. In controlled by a balance between protein factors that activate EC
contrast, telomerase activity is a common finding in many human and those that antagonize activation. Malignant tumors provide