Page 38 - eBook T2 eISBN2
P. 38
MANUAL TITLE GOES HERE
Two types of Bravais lattices will discuss here which are, cubic and hexagonal. Three types
of cubic, simple cubic, body center cubic-BCC and face center cubic-FCC. While only one
hexagonal close-packed.
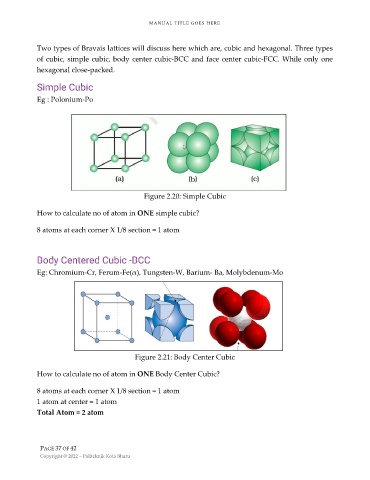
Simple Cubic
Eg : Polonium-Po
Figure 2.20: Simple Cubic
How to calculate no of atom in ONE simple cubic?
8 atoms at each corner X 1/8 section = 1 atom
Body Centered Cubic -BCC
Eg: Chromium-Cr, Ferum-Fe(α), Tungsten-W, Barium- Ba, Molybdenum-Mo
Figure 2.21: Body Center Cubic
How to calculate no of atom in ONE Body Center Cubic?
8 atoms at each corner X 1/8 section = 1 atom
1 atom at center = 1 atom
Total Atom = 2 atom
PAGE 37 OF 42
Copyright © 2022 – Politeknik Kota Bharu