Page 33 - eBook T2 eISBN2
P. 33
MANUAL TITLE GOES HERE
Crystal Solid Structure
A crystalline solid or crystal consists of atoms arranged in three dimensions in a
repetitive or uniform pattern. Crystals are composed of metals and non -metals. A single
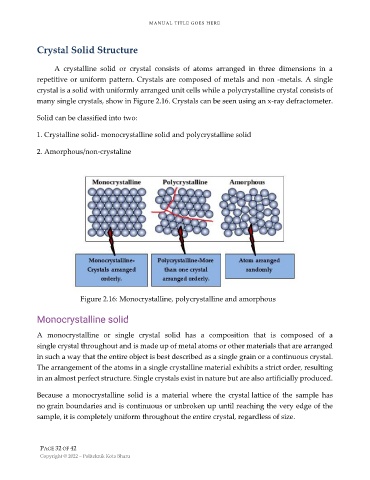
crystal is a solid with uniformly arranged unit cells while a polycrystalline crystal consists of
many single crystals, show in Figure 2.16. Crystals can be seen using an x-ray defractometer.
Solid can be classified into two:
1. Crystalline solid- monocrystalline solid and polycrystalline solid
2. Amorphous/non-crystaline
Figure 2.16: Monocrystalline, polycrystalline and amorphous
Monocrystalline solid
A monocrystalline or single crystal solid has a composition that is composed of a
single crystal throughout and is made up of metal atoms or other materials that are arranged
in such a way that the entire object is best described as a single grain or a continuous crystal.
The arrangement of the atoms in a single crystalline material exhibits a strict order, resulting
in an almost perfect structure. Single crystals exist in nature but are also artificially produced.
Because a monocrystalline solid is a material where the crystal lattice of the sample has
no grain boundaries and is continuous or unbroken up until reaching the very edge of the
sample, it is completely uniform throughout the entire crystal, regardless of size.
PAGE 32 OF 42
Copyright © 2022 – Politeknik Kota Bharu