Page 35 - eBook T2 eISBN2
P. 35
MANUAL TITLE GOES HERE
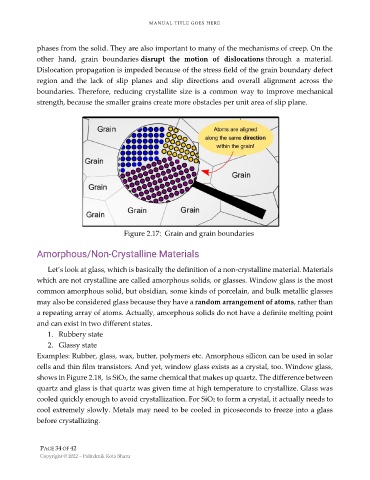
phases from the solid. They are also important to many of the mechanisms of creep. On the
other hand, grain boundaries disrupt the motion of dislocations through a material.
Dislocation propagation is impeded because of the stress field of the grain boundary defect
region and the lack of slip planes and slip directions and overall alignment across the
boundaries. Therefore, reducing crystallite size is a common way to improve mechanical
strength, because the smaller grains create more obstacles per unit area of slip plane.
Figure 2.17: Grain and grain boundaries
Amorphous/Non-Crystalline Materials
Let’s look at glass, which is basically the definition of a non-crystalline material. Materials
which are not crystalline are called amorphous solids, or glasses. Window glass is the most
common amorphous solid, but obsidian, some kinds of porcelain, and bulk metallic glasses
may also be considered glass because they have a random arrangement of atoms, rather than
a repeating array of atoms. Actually, amorphous solids do not have a definite melting point
and can exist in two different states.
1. Rubbery state
2. Glassy state
Examples: Rubber, glass, wax, butter, polymers etc. Amorphous silicon can be used in solar
cells and thin film transistors. And yet, window glass exists as a crystal, too. Window glass,
shows in Figure 2.18, is SiO2, the same chemical that makes up quartz. The difference between
quartz and glass is that quartz was given time at high temperature to crystallize. Glass was
cooled quickly enough to avoid crystallization. For SiO2 to form a crystal, it actually needs to
cool extremely slowly. Metals may need to be cooled in picoseconds to freeze into a glass
before crystallizing.
PAGE 34 OF 42
Copyright © 2022 – Politeknik Kota Bharu