Page 32 - eBook T2 eISBN2
P. 32
MANUAL TITLE GOES HERE
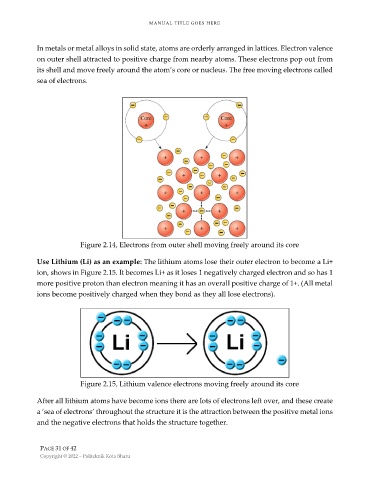
In metals or metal alloys in solid state, atoms are orderly arranged in lattices. Electron valence
on outer shell attracted to positive charge from nearby atoms. These electrons pop out from
its shell and move freely around the atom’s core or nucleus. The free moving electrons called
sea of electrons.
Figure 2.14, Electrons from outer shell moving freely around its core
Use Lithium (Li) as an example: The lithium atoms lose their outer electron to become a Li+
ion, shows in Figure 2.15. It becomes Li+ as it loses 1 negatively charged electron and so has 1
more positive proton than electron meaning it has an overall positive charge of 1+. (All metal
ions become positively charged when they bond as they all lose electrons).
Figure 2.15, Lithium valence electrons moving freely around its core
After all lithium atoms have become ions there are lots of electrons left over, and these create
a ‘sea of electrons’ throughout the structure it is the attraction between the positive metal ions
and the negative electrons that holds the structure together.
PAGE 31 OF 42
Copyright © 2022 – Politeknik Kota Bharu