Page 27 - eBook T2 eISBN2
P. 27
MANUAL TITLE GOES HERE
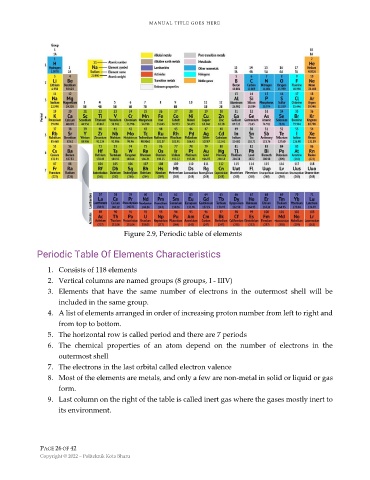
Figure 2.9, Periodic table of elements
Periodic Table Of Elements Characteristics
1. Consists of 118 elements
2. Vertical columns are named groups (8 groups, I - IIIV)
3. Elements that have the same number of electrons in the outermost shell will be
included in the same group.
4. A list of elements arranged in order of increasing proton number from left to right and
from top to bottom.
5. The horizontal row is called period and there are 7 periods
6. The chemical properties of an atom depend on the number of electrons in the
outermost shell
7. The electrons in the last orbital called electron valence
8. Most of the elements are metals, and only a few are non-metal in solid or liquid or gas
form.
9. Last column on the right of the table is called inert gas where the gases mostly inert to
its environment.
PAGE 26 OF 42
Copyright © 2022 – Politeknik Kota Bharu