Page 25 - eBook T2 eISBN2
P. 25
MANUAL TITLE GOES HERE
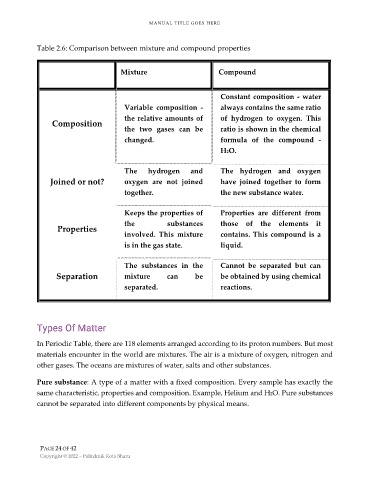
Table 2.6: Comparison between mixture and compound properties
Mixture Compound
Constant composition - water
Variable composition - always contains the same ratio
the relative amounts of of hydrogen to oxygen. This
Composition
the two gases can be ratio is shown in the chemical
changed. formula of the compound -
H2O.
The hydrogen and The hydrogen and oxygen
Joined or not? oxygen are not joined have joined together to form
together. the new substance water.
Keeps the properties of Properties are different from
the substances those of the elements it
Properties
involved. This mixture contains. This compound is a
is in the gas state. liquid.
The substances in the Cannot be separated but can
Separation mixture can be be obtained by using chemical
separated. reactions.
Types Of Matter
In Periodic Table, there are 118 elements arranged according to its proton numbers. But most
materials encounter in the world are mixtures. The air is a mixture of oxygen, nitrogen and
other gases. The oceans are mixtures of water, salts and other substances.
Pure substance: A type of a matter with a fixed composition. Every sample has exactly the
same characteristic, properties and composition. Example, Helium and H2O. Pure substances
cannot be separated into different components by physical means.
PAGE 24 OF 42
Copyright © 2022 – Politeknik Kota Bharu