Page 3 - BROCHURE 2
P. 3
PAGE 3: Keep
as it is
MEMBRANE-BASED WATER
PURIFICATION SYSTEM FOR WFI
PRODUCTION
MEMBRANE-BASED WATER
PURIFICATION SYSTEM FOR WFI
PRODUCTION
RE
Water is a substantial ingredient in the drug manufacturing process. It is REV DESCRIPTION
A REVISED PER ECO# 59712
considered a critical utility and is produced in several bulk classifications. Water
B REVISED PER ECO# 60330
for Injection (WFI) is one of these classifications described in detail within
C REVISED PER ECO# 60435
various Pharmacopeia, including the United States (US), European (Ph. Er.), Japanese D REVISED PER ECO# 60488
and Chinese Pharmacopeias. The USP, Ph. Eur. and JP revised their monographs E REVISED PER ECO# 60503
to allow for WFI production via membrane, at ambient temperatures, such as
reverse osmosis and other appropriate technologies. As an industry leader, MECO
carefully reviewed these monograph changes and designed the MECO MASTERpak™
ULTRA to produce standard WFI quality water safely and efficiently and meets
all USP, Ph. Er., and JP monograph specifications.
Water is a substantial ingredient in the drug manufacturing process. It is
considered a critical utility and is produced in several bulk classifications. Water
for Injection (WFI) is one of these classifications described in detail within
In the PH. EUR. and the USP, the major requirements on the purity of WFI are
various Pharmacopeia, including the United States (US), European (Ph. Er.),
defined as follows:
Japanese and Chinese Pharmacopeias. The USP, Ph. Eur. and JP revised
their monographs to allow for WFI production via membrane, at ambient
temperatures, such as reverse osmosis and other appropriate technologies. As
N
an industry leader, MECO carefully reviewed these monograph changes and
designed the MECO MASTERpak™ ULTRA to produce standard WFI quality
1. IT IS PREFERABLE TO LEAVE 30" [762] C
36" [914.4] CLEARANCE REQ'D IN FRONT
water safely and e ciently and meets all USP, Ph. Er., and JP monograph
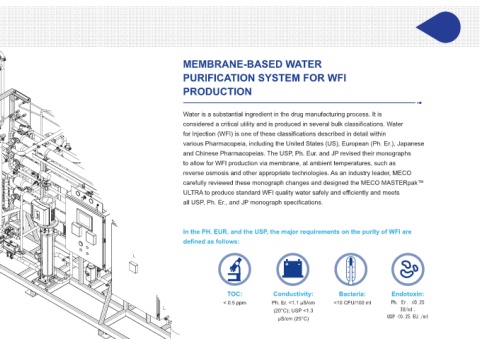
TOC: Conductivity: Bacteria: Endotoxin:
specifications.
2. CONNECTION LOCATIONS ARE ±1/4" [6.
< 0.5 ppm Ph. Er. <1.1 μS/cm <10 CFU/100 ml Ph. Er. ≤0.25 In the PH. EUR. and the USP, the major requirements on the purity of WFI are
(20°C); USP <1.3 IU/ml; defined as follows:
USP <0.25 EU./ml 3. DIMENSIONS ARE IN INCHES, [XXX] ARE
μS/cm (25°C)
TOC: Conductivity Bacteria: Endotoxin:
< 0.5 ppm : <10 CFU/100 ml Ph. Er. ≤0.25
IU/ml;
Ph. Er. <1.1 USP <0.25
µS/cm (20°C); EU./ml
USP <1.3
µS/cm (25°C)