Page 206 - The Manga Guide to Biochemistry
P. 206
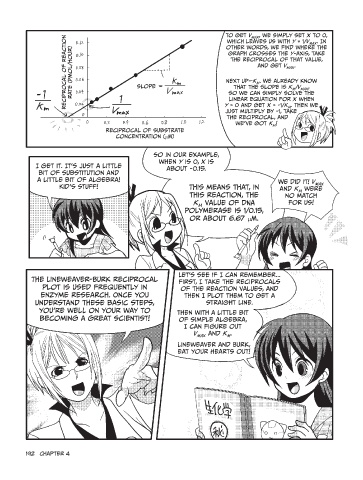
Reciprocal of reaction To get Vmax, we simply set x to 0,
rate (pMol/hour) which leaves us with y = 1/Vmax. In
other words, we find where the
graph crosses the y-axis, take
the reciprocal of that value,
and get Vmax.
slope Next up—Km. We already know
that the slope is Km/Vmax,
- so we can simply solve the
linear equation for x when
y = 0 and get x = -1/Km. Then we
just multiply by -1, take
the reciprocal, and
we’ve got Km!
Reciprocal of substrate
concentration (µM)
I get it. It’s just a little So in our example, We did it! Vmax
bit of substitution and when y is 0, x is and Km were
a little bit of algebra! about -0.15.
no match
Kid’s stuff! This means that, in for us!
this reaction, the
Km value of DNA
polymerase is 1/0.15,
or about 6.67 µM.
The Lineweaver-Burk reciprocal Let’s see if I can remember...
plot is used frequently in First, I take the reciprocals
enzyme research. Once you of the reaction values, and
understand these basic steps, then I plot them to get a
you’re well on your way to straight line.
becoming a great scientist!
Then with a little bit
of simple algebra,
I can figure out
Vmax and Km.
Lineweaver and Burk,
eat your hearts out!
192 Chapter 4