Page 113 - Medicinal Chemistry Self Assessment
P. 113
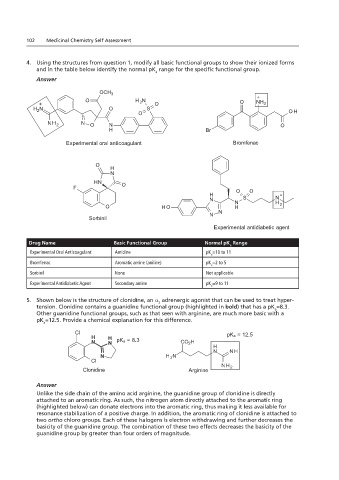
4. Using the structures from question 1, modify all basic functional groups to show their ionized forms and in the
Medicinal Chemistry Self Assessment
102
table below identify the normal pKa range for the specific functional group.
4. Using the structures from question 1, modify all basic functional groups to show their ionized forms
Answer:
and in the table below identify the normal pK range for the specific functional group.
a
Answer
OCH 3
+
O H N O NH
+ 2 O 3
H N O S OH
2
O
NH 2 N O N O
H Br
Experimental oral anticoagulant Bromfenac
O
H
N
H N O
F O O
H +
N N S N
H
O H O H 2
N N
Sorbinil
Experimental antidiabetic agent
Drug Name Basic Functional Group Normal pK Range
a
Experimental Oral Anticoagulant Amidine pK =10 to 11
a
Aromatic amine (aniline)
Bromfenac
pK =2 to 5
5. Shown below is the structure of clonidine, an D adrenergic agonist that can be used to treat hypertension.
a
Sorbinil None Not applicable
Clonidine contains a guanidine functional group (highlighted in bold) that has a pKa of 8.3. Other guanidine
Experimental Antidiabetic Agent Secondary amine pK =9 to 11
a
functional groups, such as that seen with arginine are much more basic with a pKa of 12.5. Provide a chemical
5. Shown below is the structure of clonidine, an α adrenergic agonist that can be used to treat hyper-
explanation for this difference. 2
tension. Clonidine contains a guanidine functional group (highlighted in bold) that has a pK =8.3.
a
Other guanidine functional groups, such as that seen with arginine, are much more basic with a
pK =12.5. Provide a chemical explanation for this difference.
a
pK a = 12.5
pK a = 8.3
Clonidine Arginine
Answer
Unlike the side chain of the amino acid arginine, the guanidine group of clonidine is directly
Answer:
attached to an aromatic ring. As such, the nitrogen atom directly attached to the aromatic ring
(highlighted below) can donate electrons into the aromatic ring, thus making it less available for
Unlike the side chain of the amino acid arginine, the guanidine group of clonidine is directly attached to an
resonance stabilization of a positive charge. In addition, the aromatic ring of clonidine is attached to
two ortho chloro groups. Each of these halogens is electron withdrawing and further decreases the
aromatic ring. As such, the nitrogen atom directly attached to the aromatic ring (highlighted below) can donate
basicity of the guanidine group. The combination of these two effects decreases the basicity of the
Page 3 of 5
guanidine group by greater than four orders of magnitude.
electrons into the aromatic ring, thus making it less available for resonance stabilization of a positive charge. In
addition, the aromatic ring of clonidine is attached to two ortho chloro groups. Each of these halogens is electron
withdrawing and further decreases the basicity of the guanidine group. The combination of these two effects
decreases the basicity of the guanidine group by greater than four orders of magnitude.
Involved in resonance
Cl with aromatic ring
H H
N N
N
Cl
Clonidine
Page 4 of 5