Page 108 - Medicinal Chemistry Self Assessment
P. 108
2.1 Functional Group Characteristics and Roles 97
Functional group D: This is a heterocyclic aromatic ring. It is electron withdrawing due to induction.
This electron withdrawing property is responsible for the increased acidity of the adjacent sulfon-
amide (discussed in Chapter 3*).
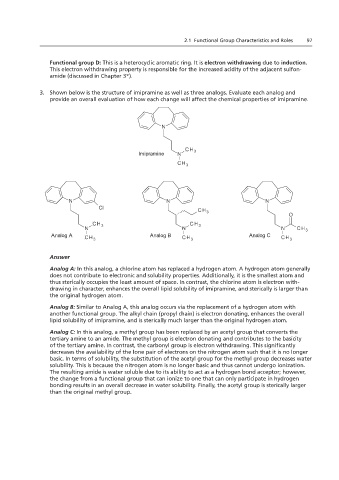
3. Shown below is the structure of imipramine as well as three analogs. Evaluate each analog and
provide an overall evaluation of how each change will affect the chemical properties of imipramine.
Imipramine
Analog A Analog B Analog C
Answer
Analog A: In this analog, a chlorine atom has replaced a hydrogen atom. A hydrogen atom generally
does not contribute to electronic and solubility properties. Additionally, it is the smallest atom and
thus sterically occupies the least amount of space. In contrast, the chlorine atom is electron with-
drawing in character, enhances the overall lipid solubility of imipramine, and sterically is larger than
the original hydrogen atom.
Analog B: Similar to Analog A, this analog occurs via the replacement of a hydrogen atom with
another functional group. The alkyl chain (propyl chain) is electron donating, enhances the overall
lipid solubility of imipramine, and is sterically much larger than the original hydrogen atom.
Analog C: In this analog, a methyl group has been replaced by an acetyl group that converts the
tertiary amine to an amide. The methyl group is electron donating and contributes to the basicity
of the tertiary amine. In contrast, the carbonyl group is electron withdrawing. This significantly
decreases the availability of the lone pair of electrons on the nitrogen atom such that it is no longer
basic. In terms of solubility, the substitution of the acetyl group for the methyl group decreases water
solubility. This is because the nitrogen atom is no longer basic and thus cannot undergo ionization.
The resulting amide is water soluble due to its ability to act as a hydrogen bond acceptor; however,
the change from a functional group that can ionize to one that can only participate in hydrogen
bonding results in an overall decrease in water solubility. Finally, the acetyl group is sterically larger
than the original methyl group.
Page 2 of 2