Page 129 - Medicinal Chemistry Self Assessment
P. 129
118 Medicinal Chemistry Self Assessment
Answer:
Answer
OH
N
O O * * *
OH
*
* *
H O
H N
Estradiol
5 chiral centers
O
Acebutolol
1 chiral center H
H 3 C N CH 3
O
H 3 CO 2 C CO 2 CH 3
* N * * S CH 3
H NO 2
OH N S N
O N
CO 2 H N N
Nifedipine
Cefamandole 0 chiral centers
3 chiral centers The highlighted carbon atom
is symmetrical
3. Shown below is the structure of fluvastatin, an HMG-CoA reductase inhibitor used to lower plasma LDL levels.
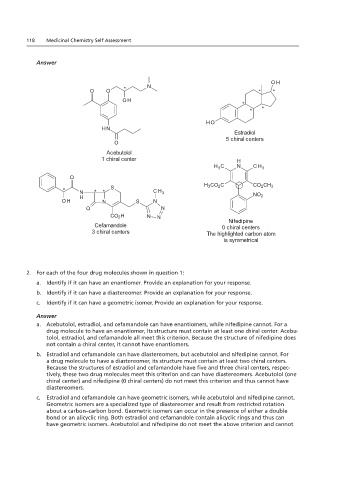
2. For each of the four drug molecules shown in question 1:
Fluvastatin contains two chiral centers, designated as A and B. Using the structure of fluvastatin and the Cahn-
a. Identify if it can have an enantiomer. Provide an explanation for your response.
Ingold-Prelog (CIP) system, determine the R/S configurations for each of its chiral centers.
b. Identify if it can have a diastereomer. Provide an explanation for your response.
c. Identify if it can have a geometric isomer. Provide an explanation for your response.
Answer H O A
CO H
a. Acebutolol, estradiol, and cefamandole can have enantiomers, while nifedipine cannot. For a
2
drug molecule to have an enantiomer, its structure must contain at least one chiral center. Acebu-
B OH
tolol, estradiol, and cefamandole all meet this criterion. Because the structure of nifedipine does
H
not contain a chiral center, it cannot have enantiomers.
b. Estradiol and cefamandole can have diastereomers, but acebutolol and nifedipine cannot. For
F
a drug molecule to have a diastereomer, its structure must contain at least two chiral centers.
N
Because the structures of estradiol and cefamandole have five and three chiral centers, respec-
tively, these two drug molecules meet this criterion and can have diastereomers. Acebutolol (one
chiral center) and nifedipine (0 chiral centers) do not meet this criterion and thus cannot have
diastereomers.
c. Estradiol and cefamandole can have geometric isomers, while acebutolol and nifedipine cannot.
Fluvastatin
Geometric isomers are a specialized type of diastereomer and result from restricted rotation
about a carbon–carbon bond. Geometric isomers can occur in the presence of either a double
bond or an alicyclic ring. Both estradiol and cefamandole contain alicyclic rings and thus can
have geometric isomers. Acebutolol and nifedipine do not meet the above criterion and cannot
Page 2 of 3