Page 142 - Medicinal Chemistry Self Assessment
P. 142
2.8 Aliskiren 131
(remove bold from drug name) Chapter 2.8
Answer
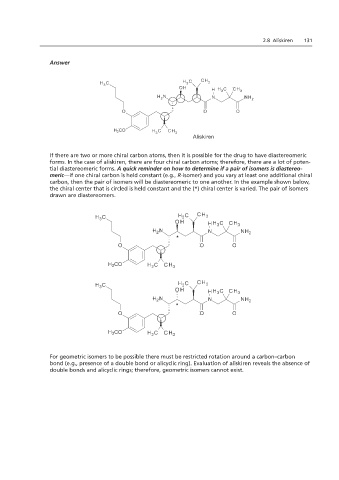
Aliskiren
If there are two or more chiral carbon atoms, then it is possible for the drug to have diastereomeric
forms. In the case of aliskiren, there are four chiral carbon atoms; therefore, there are a lot of poten-
(remove bold from drug name) Chapter 1.8/2.8
tial diastereomeric forms. A quick reminder on how to determine if a pair of isomers is diastereo-
meric—if one chiral carbon is held constant (e.g., R-isomer) and you vary at least one additional chiral
carbon, then the pair of isomers will be diastereomeric to one another. In the example shown below,
If there areheld constant and the (*) chiral center is varied. The pair of isomers drawn are diastereomers.
the chiral center that is circled is held constant and the (*) chiral center is varied. The pair of isomers
drawn are diastereomers.
*
Amlodipine
*
For geometric isomers to be possible there must be restricted rotation around a carbon–carbon
4. Approximately 25% of the absorbed dose of aliskiren is excreted in the urine unchanged. It is
bond (e.g., presence of a double bond or alicyclic ring). Evaluation of aliskiren reveals the absence of
double bonds and alicyclic rings; therefore, geometric isomers cannot exist.
transformation has occurred and whether or not it represents an oxidative transformation.
A
B