Page 74 - Medicinal Chemistry Self Assessment
P. 74
1.18 and 2.18 – remove bold from label
I
O
I
H
O
O
I H NH 2 OH
I O A
H O I
OH
H NH
I O 2 I O
I 1.18 Levothyroxine (T ) 63 I
4 O
H
OH
Levothyroxine H NH
I
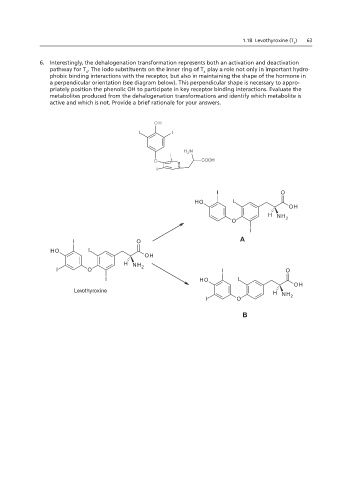
6. Interestingly, the dehalogenation transformation represents both an activation and deactivation O 2
pathway for T . The iodo substituents on the inner ring of T play a role not only in important hydro-
4
4
phobic binding interactions with the receptor, but also in maintaining the shape of the hormone in B
a perpendicular orientation (see diagram below). This perpendicular shape is necessary to appro-
priately position the phenolic OH to participate in key receptor binding interactions. Evaluate the
metabolites produced from the dehalogenation transformations and identify which metabolite is
1.18 and 2.18 – structure was fixed (pay no attention to the colors)
active and which is not. Provide a brief rationale for your answers.
1.18 and 2.18 – remove bold from label
I O
1.19 and 2.19 – remove bold from label.
H O I
OH
A H NH 2
C O
I
D
I O A
H O I
OH
H NH B
I O 2 I O
Lidocaine
I H O I
OH
Levothyroxine H NH
I O 2
B
1.18 and 2.18 – structure was fixed (pay no attention to the colors)
1.19 and 2.19 – remove bold from label.
A
C
D
B
Lidocaine