Page 78 - Medicinal Chemistry Self Assessment
P. 78
1.19 Lidocaine 67
5. Now that you have identified the metabolic transformations that generate products that have been
identified, put your detective hat on and list any additional phase I transformations that could have
occurred.
6. Lidocaine suffers from CNS-based toxicities largely due to production of the N-dealkylated metabolic
product monoethylglycinexylidide once the parent drug has crossed the blood–brain barrier.
a. Provide a structural rationale for why lidocaine is able to cross the blood–brain barrier.
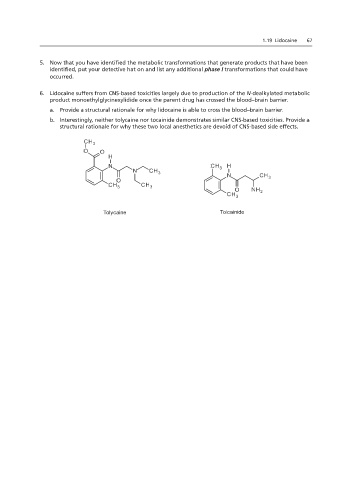
b. Interestingly, neither tolycaine nor tocainide demonstrates similar CNS-based toxicities. Provide a
1.19 and 2.19 – remove bold from label
structural rationale for why these two local anesthetics are devoid of CNS-based side effects.
Tolycaine Tolcainide
1.25 and 2.25 – remove bold from label
A
B C
D
Sitagliptin
2.25 – remove bold from label
Sitagliptin phosphate