Page 80 - Medicinal Chemistry Self Assessment
P. 80
Section 2 Whole Molecule Drug Evaluation
1.20 Montelukast and Zafirlukast
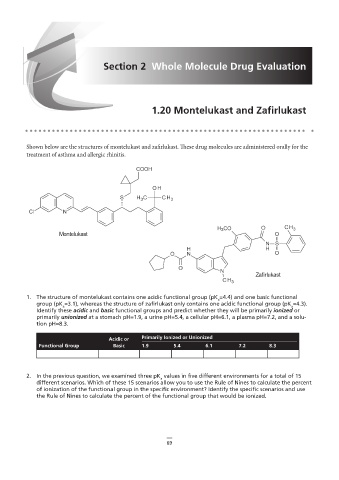
Shown below are the structures of montelukast and zafirlukast. These drug molecules are administered orally for the
treatment of asthma and allergic rhinitis.
Montelukast
Zafirlukast
1. The structure of montelukast contains one acidic functional group (pK =4.4) and one basic functional
a
group (pK =3.1), whereas the structure of zafirlukast only contains one acidic functional group (pK =4.3).
a
a
1. The structure of
Identify these acidic and basic functional groups and predict whether they will be primarily ionized or
primarily unionized at a stomach pH=1.9, a urine pH=5.4, a cellular pH=6.1, a plasma pH=7.2, and a solu-
2. In the use the Rule of Nines to calculate the percent of the functional group that would be ionized.
tion pH=8.3.
3. The an explanation for this difference.
4. Montelukast and zafirlukast. Assume that all binding interactions occur at a physiological pH of 7.4.
Primarily Ionized or Unionized
Acidic or
Functional Group Basic 1.9 5.4 6.1 7.2 8.3
5. Calculated log P these hepatic metabolism or be primarily excreted unchanged?
6. Shown below is to perform with zafirlukast.
2. In the previous question, we examined three pK values in five different environments for a total of 15
a
different scenarios. Which of these 15 scenarios allow you to use the Rule of Nines to calculate the percent
of ionization of the functional group in the specific environment? Identify the specific scenarios and use
the Rule of Nines to calculate the percent of the functional group that would be ionized.
69