Page 85 - Medicinal Chemistry Self Assessment
P. 85
1.22 Pravastatin and Fluvastatin
1.22 Pravastatin and Fluvastatin
Shown below are the structures of groups have been boxed.
Shown below are the structures of groups have been boxed.
C
B
B C D F F
D
A
A
E
E
Pravastatin
74 Medicinal Chemistry Self Assessment Fluvastatin
Pravastatin
Fluvastatin
1. Using the table below, or hydrophobic in explanation for your response.
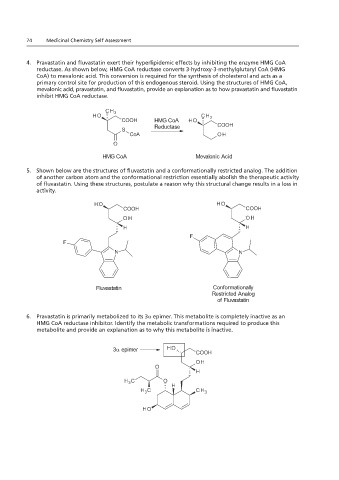
4. Pravastatin and fluvastatin exert their hyperlipidemic effects by inhibiting the enzyme HMG CoA
1. Using the table below, or hydrophobic in explanation for your response.
reductase. As shown below, HMG CoA reductase converts 3-hydroxy-3-methylglutaryl CoA (HMG
2. The log P drug a structural explanation for the difference in these log P values.
CoA) to mevalonic acid. This conversion is required for the synthesis of cholesterol and acts as a
2. The log P drug a structural explanation for the difference in these log P values.
3. The normal pK a range for normal range.
primary control site for production of this endogenous steroid. Using the structures of HMG CoA,
3. The normal pK a range for normal range.
mevalonic acid, pravastatin, and fluvastatin, provide an explanation as to how pravastatin and fluvastatin
4. Pravastatin, and as to how pravastatin and fluvastain inhibit HMG CoA reductase.
inhibit HMG CoA reductase.
4. Pravastatin, and as to how pravastatin and fluvastain inhibit HMG CoA reductase.
HMG CoA
Reductase
HMG CoA
Reductase
HMG CoA Mevalonic Acid
HMG CoA Mevalonic Acid
5. Shown below are the structures of fluvastatin and a conformationally restricted analog. The addition
5. Shown below are the postulate a reason why this structural change results in a loss in activity.
of another carbon atom and the conformational restriction essentially abolish the therapeutic activity
5. Shown below are the postulate a reason why this structural change results in a loss in activity.
of fluvastatin. Using these structures, postulate a reason why this structural change results in a loss in
activity.
Fluvastatin Conformationally
Conformationally
Fluvastatin Restricted Analog
of Fluvastatin
Restricted Analog
of Fluvastatin
6. Pravastatin is primarily metabolized to its 3α epimer. This metabolite is completely inactive as an
6. Pravastatin is is inactive.
HMG CoA reductase inhibitor. Identify the metabolic transformations required to produce this
metabolite and provide an explanation as to why this metabolite is inactive.
3D epimer