Page 87 - Medicinal Chemistry Self Assessment
P. 87
1.23 Quinapril
Shown below is are identified.
B
H 3 C
O O D
Chapter 1.23 A O
H
N
Please replace the structure for Question 2 in Chapter 1.23 with the one shown below. (NOTE: The analogous
N
76
Medicinal Chemistry Self Assessment
structure in Chapter 2.23 is fine.) C
CH 3
O OH
E
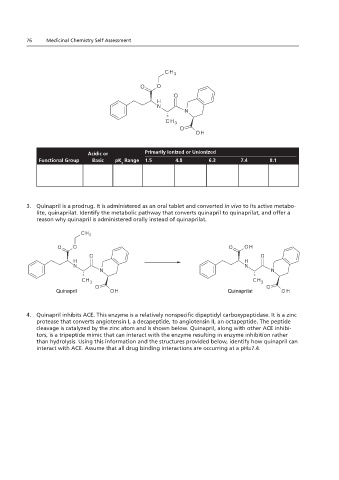
1. Using the table if it is hydrophilic or hydrophobic in brief explanation for your response.
2. Using the ionized or unionized at pH environments of 1.5, 4.8, 6.3, 7.4, and 8.1.
3.
CH 3
O O
O
H
N
N
Acidic or Primarily Ionized or Unionized
Functional Group
Chapters 1.23 and 2.23 Basic pK Range 1.5 4.8 6.3 7.4 8.1
CH
a
3
O
OH
Please replace the structures for Angiotensin I and Quinaprilat in Question 4 for both 1.23 and 2.23 with the one
provided below.
3. Quinapril is a prodrug. It is administered as an oral tablet and converted in vivo to its active metabo-
lite, quinaprilat. Identify the metabolic pathway that converts quinapril to quinaprilat, and offer a
4. Quinapril is a is administered orally instead of quinaprilat.
reason why quinapril is administered orally instead of quinaprilat.
Leu
Phe His
Quinapril Quinaprilat
Quinaprilat
Angiotensin I
R = Asp-Arg-Val-Tyr-Ile-His-Pro
4. Quinapril inhibits ACE. This enzyme is a relatively nonspecific dipeptidyl carboxypeptidase. It is a zinc
protease that converts angiotensin I, a decapeptide, to angiotensin II, an octapeptide. The peptide
cleavage is catalyzed by the zinc atom and is shown below. Quinapril, along with other ACE inhibi-
tors, is a tripeptide mimic that can interact with the enzyme resulting in enzyme inhibition rather
than hydrolysis. Using this information and the structures provided below, identify how quinapril can
interact with ACE. Assume that all drug binding interactions are occurring at a pH=7.4.