Page 146 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 146
132 SECTION II Autonomic Drugs
Imipramine, a tricyclic antidepressant drug with strong anti- the organophosphorus-cholinesterase complex if the complex has
muscarinic actions, has long been used to reduce incontinence not “aged” (see Chapter 7). Pralidoxime is the most extensively
in institutionalized elderly patients. It is moderately effective but studied—in humans—of the agents shown and the only one avail-
causes significant CNS toxicity. able for clinical use in the USA. It is most effective in regenerating
Antimuscarinic agents have also been used in urolithiasis to the cholinesterase associated with skeletal muscle neuromuscular
relieve the painful ureteral smooth muscle spasm caused by pas- junctions. Pralidoxime and obidoxime are ineffective in reversing
sage of the stone. However, their usefulness in this condition is the central effects of organophosphate poisoning because each has
debatable. positively charged quaternary ammonium groups that prevent
entry into the CNS. Diacetylmonoxime, on the other hand,
G. Cholinergic Poisoning crosses the blood-brain barrier and, in experimental animals, can
Severe cholinergic excess is a medical emergency, especially in rural regenerate some of the CNS cholinesterase.
communities where cholinesterase inhibitor insecticides are com- Pralidoxime is administered by intravenous infusion, 1–2 g
monly used and in cultures where wild mushrooms are frequently given over 15–30 minutes. In spite of the likelihood of aging
eaten. The potential use of cholinesterase inhibitors as chemical of the phosphate-enzyme complex, recent reports suggest that
warfare “nerve gases” also requires an awareness of the methods administration of multiple doses of pralidoxime over several days
for treating acute poisoning (see Chapter 58). may be useful in severe poisoning. In excessive doses, pralidoxime
can induce neuromuscular weakness and other adverse effects.
1. Antimuscarinic therapy—Both the nicotinic and the mus- Pralidoxime is not recommended for the reversal of inhibition of
carinic effects of the cholinesterase inhibitors can be life-threat- acetylcholinesterase by carbamate inhibitors. Further details of
ening. Unfortunately, there is no effective method for directly treatment of anticholinesterase toxicity are given in Chapter 58.
blocking the nicotinic effects of cholinesterase inhibition, because A third approach to protection against excessive acetylcholines-
nicotinic agonists and antagonists cause blockade of transmission terase inhibition is pretreatment with intermediate-acting enzyme
(see Chapter 27). To reverse the muscarinic effects, a tertiary (not inhibitors that transiently occupy the active site to prevent bind-
quaternary) amine drug must be used (preferably atropine) to treat ing of the much longer-acting organophosphate inhibitor. This
the CNS effects as well as the peripheral effects of the organophos- prophylaxis can be achieved with pyridostigmine but is reserved
phate inhibitors. Large doses of atropine may be needed to oppose for situations in which possibly lethal poisoning is anticipated, eg,
the muscarinic effects of extremely potent agents like parathion chemical warfare (see Chapter 7). Simultaneous use of atropine is
and chemical warfare nerve gases: 1–2 mg of atropine sulfate may required to control muscarinic excess.
be given intravenously every 5–15 minutes until signs of effect The use of biological scavengers has emerged as an adjunct
(dry mouth, reversal of miosis) appear. The drug may have to to oximes in the reactivation of acetylcholinesterase inactivated
be given many times, since the acute effects of the cholinesterase by organophosphates. Human acetylcholinesterase, acting cata-
inhibitor may last 24–48 hours or longer. In this life-threatening lytically, increased the effectiveness of PAM in reactivating the
situation, as much as 1 g of atropine per day may be required for enzyme. Butyrylcholinesterase can achieve the same effect, but
as long as 1 month for full control of muscarinic excess. it acts stoichiometrically, and thus large amounts of this bioscav-
enger are required. (Another use for butyrylcholinesterase is in
2. Cholinesterase regenerator compounds—A second class the treatment of cocaine toxicity because butyrylcholinesterase
of compounds, composed of substituted oximes capable of regen- displays cocaine hydrolase activity. The catalytic efficiency of
erating active enzyme from the organophosphorus-cholinesterase human butyrylcholinesterase against cocaine has been increased
complex, is also available to treat organophosphorus poisoning. by mutation of the enzyme such that it can prevent the effect of a
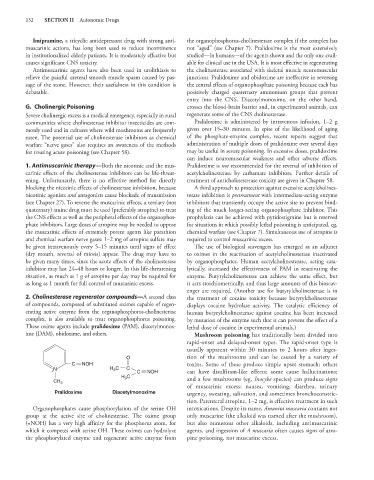
These oxime agents include pralidoxime (PAM), diacetylmonox- lethal dose of cocaine in experimental animals.)
ime (DAM), obidoxime, and others. Mushroom poisoning has traditionally been divided into
rapid-onset and delayed-onset types. The rapid-onset type is
usually apparent within 30 minutes to 2 hours after inges-
O tion of the mushrooms and can be caused by a variety of
C NOH toxins. Some of these produce simple upset stomach; others
3
+ N H C C C NOH can have disulfiram-like effects; some cause hallucinations;
H 3 C and a few mushrooms (eg, Inocybe species) can produce signs
CH 3
of muscarinic excess: nausea, vomiting, diarrhea, urinary
Pralidoxime Diacetylmonoxime urgency, sweating, salivation, and sometimes bronchoconstric-
tion. Parenteral atropine, 1–2 mg, is effective treatment in such
Organophosphates cause phosphorylation of the serine OH intoxications. Despite its name, Amanita muscaria contains not
group at the active site of cholinesterase. The oxime group only muscarine (the alkaloid was named after the mushroom),
(=NOH) has a very high affinity for the phosphorus atom, for but also numerous other alkaloids, including antimuscarinic
which it competes with serine OH. These oximes can hydrolyze agents, and ingestion of A muscaria often causes signs of atro-
the phosphorylated enzyme and regenerate active enzyme from pine poisoning, not muscarine excess.