Page 14 - Fuel Cell Teacher Edition
P. 14
• What factor most influenced the
performance of the cells? WARNiNG!
Be careful when mixing the NaOH solution.
• Design the most powerful fuel cell that When combined with water, the reaction
would fit into this form factor.
generates a great deal of heat. Slowly add
• What would happen if the fuel cells were pellets of NaOH to the water if you wish to do
connected in series? In parallel? this supplementary activity.
FUEL CELL DESIGN MODIFICATIONS ENERGY MODELING LAB PROCEDURE
When students optimize their fuel cells try to get In addition to the Fuel Cell lab, there is a
them to identify the saturation limits for copper, supplementary energy modeling lab included in this
zinc, and carbon electrode size. The saturation limit kit. The procedure is written at a college freshman
for NaOH is about 40-50%, which is too dangerous level. If your students have difficulty with this lab, there
for students to handle. As the instructor, you may is also a powerpoint presentation entitled “modeling
wish to demonstrate the concentration of this lab tutorial” which contains an in-depth explanation
amount with chemicals you have on-hand. (including pictures) of how to perform this lab.
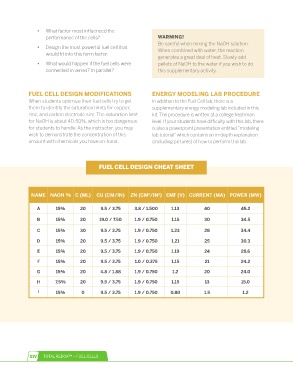
FUEL CELL DESiGN CHEAT SHEET
NAME NAOH % C (ML) CU (CM/IN) ZN (CM /IN ) EMF (V) CURRENT (MA) POWER (MW)
2
2
A 15% 20 9.5 / 3.75 3.8 / 1.500 1.13 40 45.2
B 15% 20 19.0 / 7.50 1.9 / 0.750 1.15 30 34.5
C 15% 30 9.5 / 3.75 1.9 / 0.750 1.23 28 34.4
D 15% 20 9.5 / 3.75 1.9 / 0.750 1.21 25 30.3
E 15% 20 9.5 / 3.75 1.9 / 0.750 1.19 24 28.6
F 15% 20 9.5 / 3.75 1.0 / 0.375 1.15 21 24.2
G 15% 20 4.8 / 1.88 1.9 / 0.750 1.2 20 24.0
H 7.5% 20 9.5 / 3.75 1.9 / 0.750 1.15 13 15.0
I 15% 0 9.5 / 3.75 1.9 / 0.750 0.80 1.5 1.2
Xiv TOTAL REDOX™ – FUEL CELLS TOTAL REDOX™ – FUEL CELLS Xv