Page 57 - 2021電子物理系系刊
P. 57
專題分享:張⽂豪教授實驗室
Large-area two-dimensional MoS2 growth and
transfer method
電物系 ⽑柏森 0712022
I. Introduction
Low-pressure chemical vapor deposition (CVD) is used to grow a monolayer and a
large area MoS2 at high temperatures on Aluminium oxide (Al2O3) substrate. We find that
the shape of MoS domains is highly dependent on the spatial location on the substrate,
2
with variation from a large area of film to single triangle flakes. Furthermore, under the
same spatial conditions, the heating temperature and heating time of sulfur, MoO3
precursor and substrate are closely related to the growth of MoS2.
For our growing MoS2, we encountered many problems during the transfer. In our
process, we can grow highly aligned MoS2 flakes at good growing conditions. This is of
great help in optical measurement, analysis, and component production, moreover, those
great TMDs have great performance in PL and Raman measurement . But in many time,
those 2D materials are difficult to transfer. So we use lots of transfer methods in order to
solve the problem of 2D material exfoliation.
II. Experiments
A. MoS2 growth
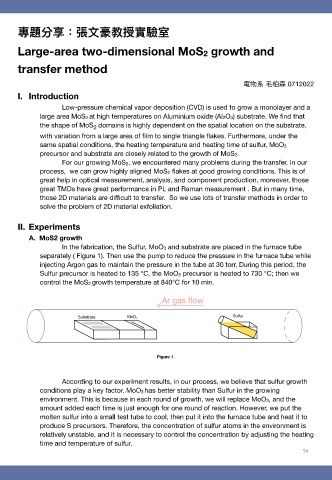
In the fabrication, the Sulfur, MoO3 and substrate are placed in the furnace tube
separately ( Figure 1). Then use the pump to reduce the pressure in the furnace tube while
injecting Argon gas to maintain the pressure in the tube at 30 torr. During this period, the
Sulfur precursor is heated to 135 °C, the MoO3 precursor is heated to 730 °C; then we
control the MoS2 growth temperature at 840°C for 10 min.
Figure 1.
According to our experiment results, in our process, we believe that sulfur growth
conditions play a key factor. MoO3 has better stability than Sulfur in the growing
environment. This is because in each round of growth, we will replace MoO3, and the
amount added each time is just enough for one round of reaction. However, we put the
molten sulfur into a small test tube to cool, then put it into the furnace tube and heat it to
produce S precursors. Therefore, the concentration of sulfur atoms in the environment is
relatively unstable, and it is necessary to control the concentration by adjusting the heating
time and temperature of sulfur.
54