Page 111 - phytochemistry I - PharmD Clinical
P. 111
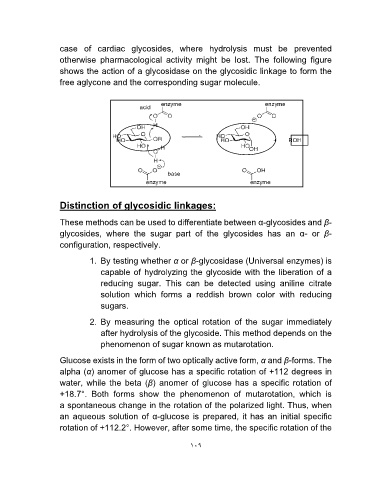
case of cardiac glycosides, where hydrolysis must be prevented
otherwise pharmacological activity might be lost. The following figure
shows the action of a glycosidase on the glycosidic linkage to form the
free aglycone and the corresponding sugar molecule.
Distinction of glycosidic linkages:
These methods can be used to differentiate between α-glycosides and β-
glycosides, where the sugar part of the glycosides has an α- or β-
configuration, respectively.
1. By testing whether α or β-glycosidase (Universal enzymes) is
capable of hydrolyzing the glycoside with the liberation of a
reducing sugar. This can be detected using aniline citrate
solution which forms a reddish brown color with reducing
sugars.
2. By measuring the optical rotation of the sugar immediately
after hydrolysis of the glycoside. This method depends on the
phenomenon of sugar known as mutarotation.
Glucose exists in the form of two optically active form, α and β-forms. The
alpha (α) anomer of glucose has a specific rotation of +112 degrees in
water, while the beta (β) anomer of glucose has a specific rotation of
+18.7°. Both forms show the phenomenon of mutarotation, which is
a spontaneous change in the rotation of the polarized light. Thus, when
an aqueous solution of α-glucose is prepared, it has an initial specific
rotation of +112.2°. However, after some time, the specific rotation of the
۱۰۹