Page 122 - Bahan Ajar Kelas XI S.1 oke pdf
P. 122
Modul Kimia Berbasis Higher Order Thinking
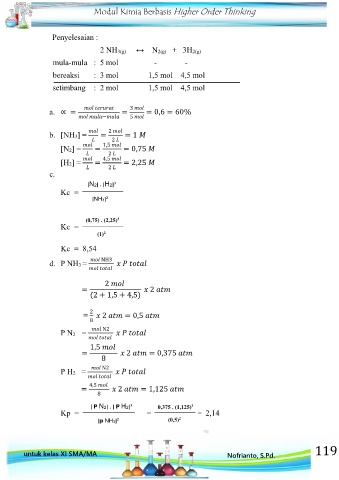
Penyelesaian :
2 NH3(g) ↔ N2(g) + 3H2(g)
mula-mula : 5 mol - -
bereaksi : 3 mol 1,5 mol 4,5 mol
setimbang : 2 mol 1,5 mol 4,5 mol
3
a. ∝ = = = 0,6 = 60%
− 5
b. [NH3] = = 2 = 1
2
[N2] = = 1,5 = 0,75
2
[H2] = = 4,5 = 2,25
2
c.
[N2] . [H2] 3
Kc =
[NH3] 2
(0,75) . (2,25) 3
Kc =
(1) 2
Kc = 8,54
d. P NH3 = NH3
2
= 2
(2 + 1,5 + 4,5)
= 2 2 = 0,5
8
P N2 = N2
1,5
= 2 = 0,375
8
P H2 = N2
4,5
= 2 = 1,125
8
[ P N2] . [ P H2] 3 0,375 . (1,125) 3
Kp = = = 2,14
[p NH3] 2 (0,5) 2
119
untuk kelas XI SMA/MA Nofrianto, S.Pd. 1 1 9