Page 359 - Copper and Bronze in Art: Corrosion, Colorants, Getty Museum Conservation, By David Scott
P. 359
[I]f this proceeds, the copper is dissolved by carbon dioxide containing waters, so that
finally pure tin oxide remains which combines with various compounds from the soil
[T]he degree of this decomposition depends on the ratio of oxygen and aggressive car
bon dioxide in the groundwaters. If oxygen is missing the decomposition will be slow,
even in the presence of high amounts of carbon dioxide.... [I] f there is a lot of oxygen, but
little carbon dioxide, then the metal is often oxidized and covered with a carbonate patina.
(Geilmann 1956:210)
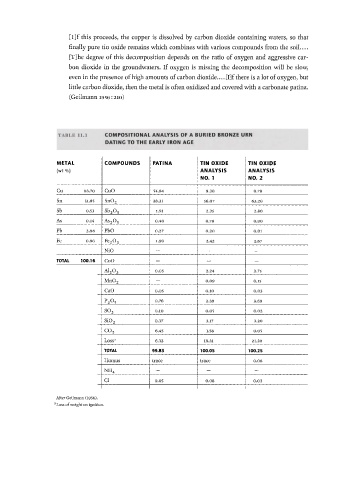
TABLE l l . l COMPOSITIONAL ANALYSIS OF A BURIED BRONZE URN
DATING TO THE EARLY IRON ACE
METAL COMPOUNDS PATINA TIN OXIDE TIN OXIDE
(wt %) ANALYSIS ANALYSIS
NO. 1 NO. 2
Cu 83.70 CuO 54.84 9.30 0.78
Sn 11.85 Sn0 2 28.31 56.07 63.20
Sb 0.53 s b 2 o 5 1.91 2.35 2.80
As 0.14 As 2 0 5 0.40 0.78 0.90
Pb 2.98 PbO 0.27 0.20 0.01
Fe 0.96 Fe 2 0 3 1.90 2.42 2.67
NiO — — —
TOTAL 100.16 CoO - — —
0.05 2.24 2.75
A1 2 0 3
— 0.09 0.15
Mn0 2
CaO 0.05 0.10 0.03
0.76 2.39 2.69
P 2 O 5
0.10 0.05 0.02
S0 3
0.37 3.17 3.20
s i o 2
c o 6.45 1.58 0.05
2
Loss 3 6.32 19.31 21.30
TOTAL 99.83 100.05 100.25
Humus trace trace 0.08
- - —
NH 4
CI 0.05 0.08 0.03
After Geilmann (1956).
Loss of weight on ignition.
l