Page 360 - Copper and Bronze in Art: Corrosion, Colorants, Getty Museum Conservation, By David Scott
P. 360
These corrosion processes, Geilmann argues, occur in the absence of chloride ions, and his
analytical results tend to support this interpretation. The presence of humic acids in the soil—
along with enhanced levels of soil minerals such as iron, silicon, and aluminum—was also
shown to be an important factor in the corrosion of the bronze objects from these sites.
Several papers have confirmed the essential fact of humic acid's role in the corrosion of
buried copper-alloy objects. Taube, King, and Chase (1997), for example, found that Chinese
bronze mirrors with a typical composition of 70Cu25Sn5Pb might have a 100 μπι thick patina
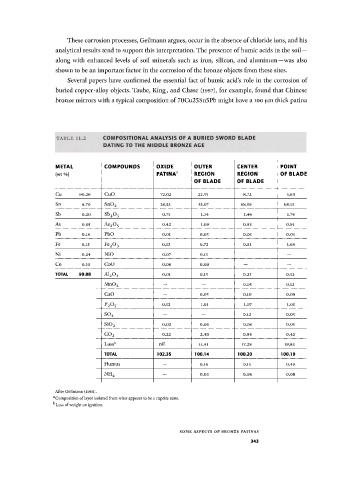
TABLE 11.2 C O M P O S I T I O N A L A N A L Y S I S O F A B U R I E D S W O R D B L A D E
D A T I N G T O T H E M I D D L E B R O N Z E A G E
M E T A L C O M P O U N D S O X I D E O U T E R C E N T E R P O I N T
(wt %) PATINA 3 R E G I O N R E G I O N O F B L A D E
O F B L A D E O F B L A D E
Cu 90.26 CuO 72.02 22.55 8.72 5.60
Sn 8.70 Sn0 2 28.53 55.07 68.59 68.15
Sb 0.20 s b 2 o 5 0.75 1.34 1.46 1.74
As 0.05 As 2 0 5 0.42 1.09 0.93 0.91
Pb 0.I8 PbO 0.01 0.05 0.05 0.05
Fe 0.15 Fe 2 0 3 0.12 0.72 0.91 1.64
Ni 0.24 NiO 0.07 0.15 — —
Co 0.10 CoO 0.06 0.09 — —
TOTAL 99.88 A1 2 0 3 0.01 0.15 0.25 0.52
— — 0.05 0.12
Mn0 2
CaO — 0.05 0.10 0.09
0.12 1.01 1.07 1.05
P 2 O 5
— — 0.12 0.05
s o 3
0.02 0.06 0.08 0.05
Si0 2
0.22 2.40 0.64 0.42
c o 2
Loss b nil 15.41 17.28 19.83
TOTAL 102.35 100.14 100.20 100.19
Humus — 0.16 0.15 0.49
— 0.03 0.06 0.08
NH 4
After Geilmann (1956).
Composition of layer isolated from what appears to be a cuprite zone.
1
' Loss of weight on ignition.
S O M E A S P E C T S O F B R O N Z E PATINA S
343