Page 148 - pfizervax
P. 148
PF-07302048 (BNT162 RNA-Based COVID-19 Vaccines)
Protocol C4591001
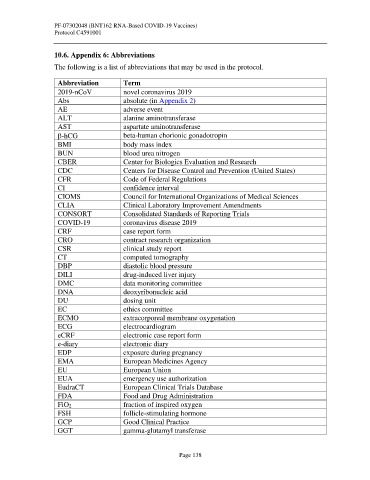
10.6. Appendix 6: Abbreviations
The following is a list of abbreviations that may be used in the protocol.
Abbreviation Term
2019-nCoV novel coronavirus 2019
Abs absolute (in Appendix 2)
AE adverse event
ALT alanine aminotransferase
AST aspartate aminotransferase
-hCG beta-human chorionic gonadotropin
BMI body mass index
BUN blood urea nitrogen
CBER Center for Biologics Evaluation and Research
CDC Centers for Disease Control and Prevention (United States)
CFR Code of Federal Regulations
CI confidence interval
CIOMS Council for International Organizations of Medical Sciences
CLIA Clinical Laboratory Improvement Amendments
CONSORT Consolidated Standards of Reporting Trials
COVID-19 coronavirus disease 2019
CRF case report form
CRO contract research organization
CSR clinical study report
CT computed tomography
DBP diastolic blood pressure
DILI drug-induced liver injury
DMC data monitoring committee
DNA deoxyribonucleic acid
DU dosing unit
EC ethics committee
ECMO extracorporeal membrane oxygenation
ECG electrocardiogram
eCRF electronic case report form
e-diary electronic diary
EDP exposure during pregnancy
EMA European Medicines Agency
EU European Union
EUA emergency use authorization
EudraCT European Clinical Trials Database
FDA Food and Drug Administration
FiO2 fraction of inspired oxygen
FSH follicle-stimulating hormone
GCP Good Clinical Practice
GGT gamma-glutamyl transferase
Page 138