Page 469 - Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice
P. 469
Fluid, Electrolyte, and Acid-Base Disturbances in Liver Disease 457
Monovalent Divalent
bile salt conjugated Vesicle mediated
export pump export pump canalicular
MRP2 bile salt transport
Bile salt
Na /bile salt BS
Co-transporter Bile salt
Na
BS Na /bile salt
BS BS Co-transporter
Paracellular Na Na
(diffusion) H 2 O Na H 2 O
Na Na Canalicular secretion
Bile salt dependent
HCO 3
Regulated by bile acid load
HCO 3 HCO 3 Carbonic direct vs. vesicle mediated,
Na Na anhydrase accounts for 30–60% basal
bile flow
H H H 2 O CO 2 Bile salt-independent
Glutathione Bile salts Regulated by hormones
mediated ATP (e.g., glucagon)
reflects ion transport
BS
Paracellular mediated:
(diffusion) GSH dominantly by GSH,
inorganic electrolytes
H 2 O
Na account for 30–60%
Vesicle mediated H 2 O Na
Na basal bile flow
Cl
Secretin Na H 2 O HCO 3 Cl Ductular secretion
cAMP H 2 O Regulated by secretin,
Cl Cl influences alkalinization and
dilution of bile: (HCO 3 , Cl )
Cl
HCO 3 HCO 3
HCO 3 Cl
Bile salts
Cholesterol
Polyunsaturated
Phosphatidylcholine BS = Bile salt
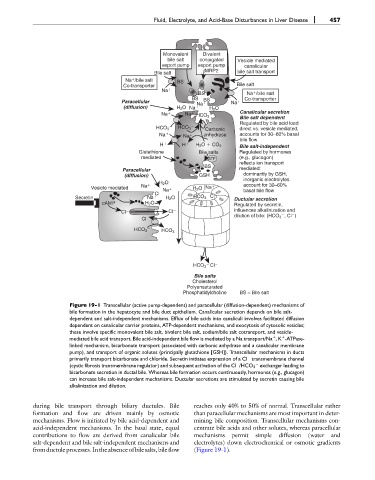
Figure 19-1 Transcellular (active pump-dependent) and paracellular (diffusion-dependent) mechanisms of
bile formation in the hepatocyte and bile duct epithelium. Canalicular secretion depends on bile salt-
dependent and salt-independent mechanisms. Efflux of bile acids into canaliculi involves facilitated diffusion
dependent on canalicular carrier proteins, ATP-dependent mechanisms, and exocytosis of cytosolic vesicles;
these involve specific monovalent bile salt, bivalent bile salt, sodium/bile salt cotransport, and vesicle-
mediated bile acid transport. Bile acid-independent bile flow is mediated by a Na transport/Na ,K -ATPase-
þ
þ
linked mechanism, bicarbonate transport (associated with carbonic anhydrase and a canalicular membrane
pump), and transport of organic solutes (principally glutathione [GSH]). Transcellular mechanisms in ducts
primarily transport bicarbonate and chloride. Secretin initiates expression of a Cl transmembrane channel
(cystic fibrosis transmembrane regulator) and subsequent activation of the Cl /HCO 3 exchanger leading to
bicarbonate secretion in ductal bile. Whereas bile formation occurs continuously, hormones (e.g., glucagon)
can increase bile salt-independent mechanisms. Ductular secretions are stimulated by secretin causing bile
alkalinization and dilution.
during bile transport through biliary ductules. Bile reaches only 40% to 50% of normal. Transcellular rather
formation and flow are driven mainly by osmotic than paracellular mechanisms are most important in deter-
mechanisms. Flow is initiated by bile acid-dependent and mining bile composition. Transcellular mechanisms con-
acid-independent mechanisms. In the basal state, equal centrate bile acids and other solutes, whereas paracellular
contributions to flow are derived from canalicular bile mechanisms permit simple diffusion (water and
salt-dependent and bile salt-independent mechanisms and electrolytes) down electrochemical or osmotic gradients
fromductule processes.Intheabsenceofbilesalts,bileflow (Figure 19-1).