Page 324 - The Veterinary Laboratory and Field Manual 3rd Edition
P. 324
Haematology 293
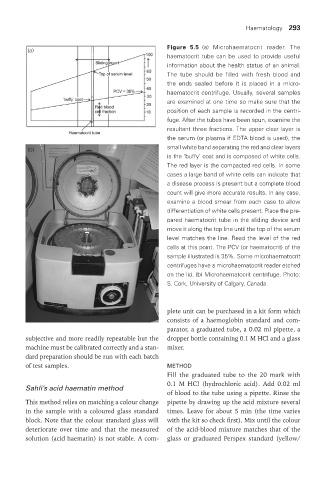
Figure 5.5 (a) Microhaematocrit reader. The
(a)
haematocrit tube can be used to provide useful
information about the health status of an animal.
The tube should be filled with fresh blood and
the ends sealed before it is placed in a micro-
haematocrit centrifuge. Usually, several samples
are examined at one time so make sure that the
position of each sample is recorded in the centri-
fuge. After the tubes have been spun, examine the
resultant three fractions. The upper clear layer is
the serum (or plasma if EDTA blood is used), the
(b) small white band separating the red and clear layers
is the ‘buffy’ coat and is composed of white cells.
The red layer is the compacted red cells. In some
cases a large band of white cells can indicate that
a disease process is present but a complete blood
count will give more accurate results. In any case,
examine a blood smear from each case to allow
differentiation of white cells present. Place the pre-
pared haematocrit tube in the sliding device and
move it along the top line until the top of the serum
level matches the line. Read the level of the red
cells at this point. The PCV (or haematocrit) of the
sample illustrated is 35%. Some microhaematocrit
centrifuges have a microhaematocrit reader etched
on the lid. (b) Microhaematocrit centrifuge. Photo:
S. Cork, University of Calgary, Canada.
plete unit can be purchased in a kit form which
consists of a haemoglobin standard and com-
parator, a graduated tube, a 0.02 ml pipette, a
subjective and more readily repeatable but the dropper bottle containing 0.1 M HCl and a glass
machine must be calibrated correctly and a stan- mixer.
dard preparation should be run with each batch
of test samples. MEtHod
Fill the graduated tube to the 20 mark with
0.1 M HCl (hydrochloric acid). Add 0.02 ml
Sahli’s acid haematin method
of blood to the tube using a pipette. Rinse the
This method relies on matching a colour change pipette by drawing up the acid mixture several
in the sample with a coloured glass standard times. Leave for about 5 min (the time varies
block. Note that the colour standard glass will with the kit so check first). Mix until the colour
deteriorate over time and that the measured of the acid-blood mixture matches that of the
solution (acid haematin) is not stable. A com- glass or graduated Perspex standard (yellow/
Vet Lab.indb 293 26/03/2019 10:25