Page 64 - Clinical Small Animal Internal Medicine
P. 64
32 Section 2 Endocrine Disease
200 endocrine tissue. Examples include primary hypothyroid
VetBooks.ir 175 ism in dogs and hypoadrenocorticism (Addison disease)
in dogs and cats. The immune‐mediated destruction and
150
gradual loss of hormone secretion activate negative feed
Cortisol (nmol/L) 125 Hyperadrenocorticism back mechanisms so that a period of compensation typi
cally occurs. For example, levels of TSH would be expected
100
to rise in dogs with primary hypothyroidism, possibly
75
immune‐mediated destruction results in loss of sufficient
50 Normal even before clinical signs are noted. Eventually, the
endocrine tissue so that the hormone secretion declines
25
to the point that clinical signs of deficiency are seen.
0 Often, endocrine hyperfunction conditions result
0 4 8 from overactivity of endocrine cells associated with
Hours either hyperplasia or neoplasia. Often, these affected
cells not only possess increased cell division rates but
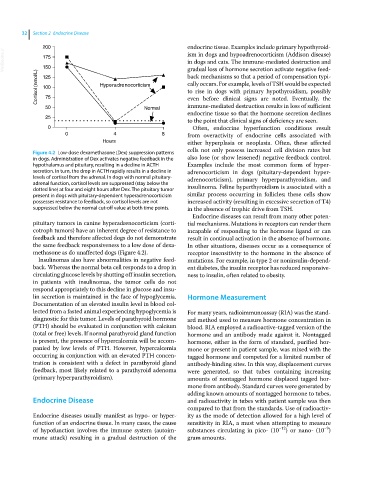
Figure 4.2 Low‐dose dexamethasone (Dex) suppression patterns
in dogs. Administration of Dex activates negative feedback in the also lose (or show lessened) negative feedback control.
hypothalamus and pituitary, resulting in a decline in ACTH Examples include the most common form of hyper
secretion. In turn, the drop in ACTH rapidly results in a decline in adrenocorticism in dogs (pituitary‐dependent hyper
levels of cortisol from the adrenal. In dogs with normal pituitary‐ adrenocorticism), primary hyperparathyroidism, and
adrenal function, cortisol levels are suppressed (stay below the
dotted line) at four and eight hours after Dex. The pituitary tumor insulinoma. Feline hyperthyroidism is associated with a
present in dogs with pituitary‐dependent hyperadrenocorticism similar process occurring in follicles; these cells show
possesses resistance to feedback, so cortisol levels are not increased activity (resulting in excessive secretion of T4)
suppressed below the normal cut‐off value at both time points. in the absence of trophic drive from TSH.
Endocrine diseases can result from many other poten
pituitary tumors in canine hyperadrenocorticism (corti tial mechanisms. Mutations in receptors can render them
cotroph tumors) have an inherent degree of resistance to incapable of responding to the hormone ligand or can
feedback and therefore affected dogs do not demonstrate result in continual activation in the absence of hormone.
the same feedback responsiveness to a low dose of dexa In other situations, diseases occur as a consequence of
methasone as do unaffected dogs (Figure 4.2). receptor insensitivity to the hormone in the absence of
Insulinomas also have abnormalities in negative feed mutations. For example, in type 2 or noninsulin‐depend
back. Whereas the normal beta cell responds to a drop in ent diabetes, the insulin receptor has reduced responsive
circulating glucose levels by shutting off insulin secretion, ness to insulin, often related to obesity.
in patients with insulinomas, the tumor cells do not
respond appropriately to this decline in glucose and insu
lin secretion is maintained in the face of hypoglycemia. Hormone Measurement
Documentation of an elevated insulin level in blood col
lected from a fasted animal experiencing hypoglycemia is For many years, radioimmunoassay (RIA) was the stand
diagnostic for this tumor. Levels of parathyroid hormone ard method used to measure hormone concentration in
(PTH) should be evaluated in conjunction with calcium blood. RIA employed a radioactive‐tagged version of the
(total or free) levels. If normal parathyroid gland function hormone and an antibody made against it. Nontagged
is present, the presence of hypercalcemia will be accom hormone, either in the form of standard, purified hor
panied by low levels of PTH. However, hypercalcemia mone or present in patient sample, was mixed with the
occurring in conjunction with an elevated PTH concen tagged hormone and competed for a limited number of
tration is consistent with a defect in parathyroid gland antibody‐binding sites. In this way, displacement curves
feedback, most likely related to a parathyroid adenoma were generated, so that tubes containing increasing
(primary hyperparathyroidism). amounts of nontagged hormone displaced tagged hor
mone from antibody. Standard curves were generated by
adding known amounts of nontagged hormone to tubes,
Endocrine Disease and radioactivity in tubes with patient sample was then
compared to that from the standards. Use of radioactiv
Endocrine diseases usually manifest as hypo‐ or hyper ity as the mode of detection allowed for a high level of
function of an endocrine tissue. In many cases, the cause sensitivity in RIA, a must when attempting to measure
−9
of hypofunction involves the immune system (autoim substances circulating in pico‐ (10 −12 ) or nano‐ (10 )
mune attack) resulting in a gradual destruction of the gram amounts.