Page 76 - Heart Transplant Protocol
P. 76
Heart Function Service: Heart Transplant Protocols
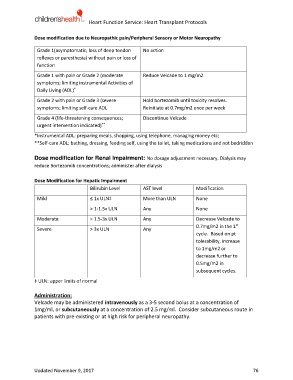
Dose modification due to Neuropathic pain/Peripheral Sensory or Motor Neuropathy
Grade 1(asymptomatic; loss of deep tendon No action
reflexes or paresthesia) without pain or loss of
function
Grade 1 with pain or Grade 2 (moderate Reduce Velcade to 1 mg/m2
symptoms; limiting instrumental Activities of
Daily Living (ADL) *
Grade 2 with pain or Grade 3 (severe Hold bortezomib until toxicity resolves.
symptoms; limiting self-care ADL Reinitiate at 0.7mg/m2 once per week
Grade 4 (life-threatening consequences; Discontinue Velcade
urgent intervention indicated) **
*Instrumental ADL: preparing meals, shopping, using telephone, managing money etc;
**Self-care ADL: bathing, dressing, feeding self, using the toilet, taking medications and not bedridden
Dose modification for Renal Impairment: No dosage adjustment necessary. Dialysis may
reduce Bortezomib concentrations; administer after dialysis
Dose Modification for Hepatic Impairment
Bilirubin Level AST level Modification
Mild ≤ 1x ULN‡ More than ULN None
˃ 1-1.5x ULN Any None
Moderate ˃ 1.5-3x ULN Any Decrease Velcade to
st
0.7mg/m2 in the 1
Severe ˃ 3x ULN Any
cycle. Based on pt
tolerability, increase
to 1mg/m2 or
decrease further to
0.5mg/m2 in
subsequent cycles.
‡ ULN: upper limits of normal
Administration:
Velcade may be administered intravenously as a 3-5 second bolus at a concentration of
1mg/ml, or subcutaneously at a concentration of 2.5 mg/ml. Consider subcutaneous route in
patients with pre-existing or at high risk for peripheral neuropathy.
Updated November 9, 2017 76