Page 1021 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 1021
CHAPTER 56 Introduction to Toxicology: Occupational & Environmental 1007
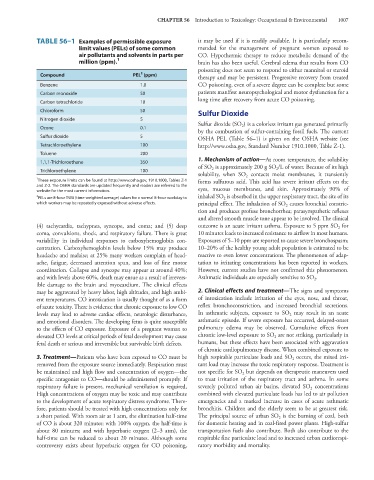
TABLE 56–1 Examples of permissible exposure it may be used if it is readily available. It is particularly recom-
limit values (PELs) of some common mended for the management of pregnant women exposed to
air pollutants and solvents in parts per CO. Hypothermic therapy to reduce metabolic demand of the
million (ppm). 1 brain has also been useful. Cerebral edema that results from CO
poisoning does not seem to respond to either mannitol or steroid
2
Compound PEL (ppm) therapy and may be persistent. Progressive recovery from treated
Benzene 1.0 CO poisoning, even of a severe degree can be complete but some
Carbon monoxide 50 patients manifest neuropsychological and motor dysfunction for a
long time after recovery from acute CO poisoning.
Carbon tetrachloride 10
Chloroform 50 Sulfur Dioxide
Nitrogen dioxide 5
Sulfur dioxide (SO ) is a colorless irritant gas generated primarily
Ozone 0.1 2
by the combustion of sulfur-containing fossil fuels. The current
Sulfur dioxide 5 OSHA PEL (Table 56–1) is given on the OSHA website (see
Tetrachloroethylene 100 http://www.osha.gov, Standard Number 1910.1000, Table Z-1).
Toluene 200
1. Mechanism of action—At room temperature, the solubility
1,1,1-Trichloroethane 350
of SO is approximately 200 g SO /L of water. Because of its high
Trichloroethylene 100 2 2
solubility, when SO contacts moist membranes, it transiently
2
1 These exposure limits can be found at http://www.osha.gov, 1910.1000, Tables Z-1 forms sulfurous acid. This acid has severe irritant effects on the
and Z-2. The OSHA standards are updated frequently and readers are referred to the eyes, mucous membranes, and skin. Approximately 90% of
website for the most current information.
2 inhaled SO is absorbed in the upper respiratory tract, the site of its
PELs are 8-hour TWA (time-weighted average) values for a normal 8-hour workday to 2
which workers may be repeatedly exposed without adverse effects. principal effect. The inhalation of SO causes bronchial constric-
2
tion and produces profuse bronchorrhea; parasympathetic reflexes
and altered smooth muscle tone appear to be involved. The clinical
(4) tachycardia, tachypnea, syncope, and coma; and (5) deep outcome is an acute irritant asthma. Exposure to 5 ppm SO for
2
coma, convulsions, shock, and respiratory failure. There is great 10 minutes leads to increased resistance to airflow in most humans.
variability in individual responses to carboxyhemoglobin con- Exposures of 5–10 ppm are reported to cause severe bronchospasm;
centration. Carboxyhemoglobin levels below 15% may produce 10–20% of the healthy young adult population is estimated to be
headache and malaise; at 25% many workers complain of head- reactive to even lower concentrations. The phenomenon of adap-
ache, fatigue, decreased attention span, and loss of fine motor tation to irritating concentrations has been reported in workers.
coordination. Collapse and syncope may appear at around 40%; However, current studies have not confirmed this phenomenon.
and with levels above 60%, death may ensue as a result of irrevers- Asthmatic individuals are especially sensitive to SO .
2
ible damage to the brain and myocardium. The clinical effects
may be aggravated by heavy labor, high altitudes, and high ambi- 2. Clinical effects and treatment—The signs and symptoms
ent temperatures. CO intoxication is usually thought of as a form of intoxication include irritation of the eyes, nose, and throat,
of acute toxicity. There is evidence that chronic exposure to low CO reflex bronchoconstriction, and increased bronchial secretions.
levels may lead to adverse cardiac effects, neurologic disturbance, In asthmatic subjects, exposure to SO may result in an acute
2
and emotional disorders. The developing fetus is quite susceptible asthmatic episode. If severe exposure has occurred, delayed-onset
to the effects of CO exposure. Exposure of a pregnant woman to pulmonary edema may be observed. Cumulative effects from
elevated CO levels at critical periods of fetal development may cause chronic low-level exposure to SO are not striking, particularly in
2
fetal death or serious and irreversible but survivable birth defects. humans, but these effects have been associated with aggravation
of chronic cardiopulmonary disease. When combined exposure to
3. Treatment—Patients who have been exposed to CO must be high respirable particulate loads and SO 2 occurs, the mixed irri-
removed from the exposure source immediately. Respiration must tant load may increase the toxic respiratory response. Treatment is
be maintained and high flow and concentration of oxygen—the not specific for SO but depends on therapeutic maneuvers used
2
specific antagonist to CO—should be administered promptly. If to treat irritation of the respiratory tract and asthma. In some
respiratory failure is present, mechanical ventilation is required, severely polluted urban air basins, elevated SO concentrations
2
High concentrations of oxygen may be toxic and may contribute combined with elevated particulate loads has led to air pollution
to the development of acute respiratory distress syndrome. There- emergencies and a marked increase in cases of acute asthmatic
fore, patients should be treated with high concentrations only for bronchitis. Children and the elderly seem to be at greatest risk.
a short period. With room air at 1 atm, the elimination half-time The principal source of urban SO is the burning of coal, both
2
of CO is about 320 minutes; with 100% oxygen, the half-time is for domestic heating and in coal-fired power plants. High-sulfur
about 80 minutes; and with hyperbaric oxygen (2–3 atm), the transportation fuels also contribute. Both also contribute to the
half-time can be reduced to about 20 minutes. Although some respirable fine particulate load and to increased urban cardiorespi-
controversy exists about hyperbaric oxygen for CO poisoning, ratory morbidity and mortality.