Page 1066 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 1066
1052 SECTION X Special Topics
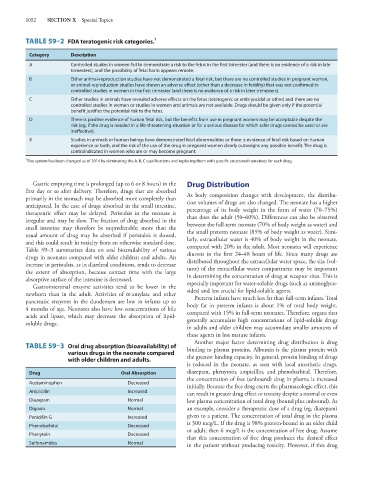
TABLE 59–2 FDA teratogenic risk categories. 1
Category Description
A Controlled studies in women fail to demonstrate a risk to the fetus in the first trimester (and there is no evidence of a risk in late
trimesters), and the possibility of fetal harm appears remote.
B Either animal-reproduction studies have not demonstrated a fetal risk, but there are no controlled studies in pregnant women,
or animal-reproduction studies have shown an adverse effect (other than a decrease in fertility) that was not confirmed in
controlled studies in women in the first trimester (and there is no evidence of a risk in later trimesters).
C Either studies in animals have revealed adverse effects on the fetus (teratogenic or embryocidal or other) and there are no
controlled studies in women or studies in women and animals are not available. Drugs should be given only if the potential
benefit justifies the potential risk to the fetus.
D There is positive evidence of human fetal risk, but the benefits from use in pregnant women may be acceptable despite the
risk (eg, if the drug is needed in a life-threatening situation or for a serious disease for which safer drugs cannot be used or are
ineffective).
X Studies in animals or human beings have demonstrated fetal abnormalities or there is evidence of fetal risk based on human
experience or both, and the risk of the use of the drug in pregnant women clearly outweighs any possible benefit. The drug is
contraindicated in women who are or may become pregnant.
1
This system has been changed as of 2014 by eliminating the A, B, C qualifications and replacing them with specific structured narratives for each drug.
Gastric emptying time is prolonged (up to 6 or 8 hours) in the Drug Distribution
first day or so after delivery. Therefore, drugs that are absorbed As body composition changes with development, the distribu-
primarily in the stomach may be absorbed more completely than tion volumes of drugs are also changed. The neonate has a higher
anticipated. In the case of drugs absorbed in the small intestine, percentage of its body weight in the form of water (70–75%)
therapeutic effect may be delayed. Peristalsis in the neonate is than does the adult (50–60%). Differences can also be observed
irregular and may be slow. The fraction of drug absorbed in the between the full-term neonate (70% of body weight as water) and
small intestine may therefore be unpredictable; more than the the small preterm neonate (85% of body weight as water). Simi-
usual amount of drug may be absorbed if peristalsis is slowed, larly, extracellular water is 40% of body weight in the neonate,
and this could result in toxicity from an otherwise standard dose. compared with 20% in the adult. Most neonates will experience
Table 59–3 summarizes data on oral bioavailability of various diuresis in the first 24–48 hours of life. Since many drugs are
drugs in neonates compared with older children and adults. An distributed throughout the extracellular water space, the size (vol-
increase in peristalsis, as in diarrheal conditions, tends to decrease ume) of the extracellular water compartment may be important
the extent of absorption, because contact time with the large in determining the concentration of drug at receptor sites. This is
absorptive surface of the intestine is decreased. especially important for water-soluble drugs (such as aminoglyco-
Gastrointestinal enzyme activities tend to be lower in the
newborn than in the adult. Activities of α-amylase and other sides) and less crucial for lipid-soluble agents.
Preterm infants have much less fat than full-term infants. Total
pancreatic enzymes in the duodenum are low in infants up to body fat in preterm infants is about 1% of total body weight,
4 months of age. Neonates also have low concentrations of bile compared with 15% in full-term neonates. Therefore, organs that
acids and lipase, which may decrease the absorption of lipid- generally accumulate high concentrations of lipid-soluble drugs
soluble drugs.
in adults and older children may accumulate smaller amounts of
these agents in less mature infants.
Another major factor determining drug distribution is drug
TABLE 59–3 Oral drug absorption (bioavailability) of binding to plasma proteins. Albumin is the plasma protein with
various drugs in the neonate compared
with older children and adults. the greatest binding capacity. In general, protein binding of drugs
is reduced in the neonate, as seen with local anesthetic drugs,
Drug Oral Absorption diazepam, phenytoin, ampicillin, and phenobarbital. Therefore,
the concentration of free (unbound) drug in plasma is increased
Acetaminophen Decreased
initially. Because the free drug exerts the pharmacologic effect, this
Ampicillin Increased can result in greater drug effect or toxicity despite a normal or even
Diazepam Normal low plasma concentration of total drug (bound plus unbound). As
Digoxin Normal an example, consider a therapeutic dose of a drug (eg, diazepam)
Penicillin G Increased given to a patient. The concentration of total drug in the plasma
is 300 mcg/L. If the drug is 98% protein-bound in an older child
Phenobarbital Decreased
or adult, then 6 mcg/L is the concentration of free drug. Assume
Phenytoin Decreased
that this concentration of free drug produces the desired effect
Sulfonamides Normal in the patient without producing toxicity. However, if this drug