Page 1192 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 1192
All adults; pregnant women should receive a dose with each
pregnancy (preferred during 27–36 weeks of gestation)
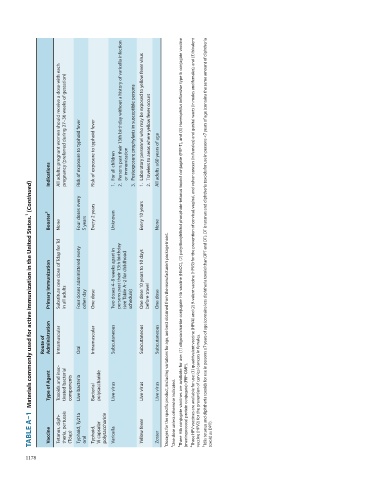
Risk of exposure to typhoid fever Risk of exposure to typhoid fever Persons past their 13th birthday without a history of varicella infection Postexposure prophylaxis in susceptible persons Laboratory personnel who may be exposed to yellow fever virus Travelers to areas where yellow fever occurs All adults ≥60 years of age
Indications For all children 1. 2. or immunization 3. 1. 2.
Materials commonly used for active immunization in the United States. 1 (Continued)
Booster 2 None Four doses every 5 years Every 2 years Unknown Every 10 years None 3 Three Hib conjugate vaccines are available for use: (1) oligosaccharide conjugate Hib vaccine (HbOC), (2) polyribosylribitol phosphate-tetanus toxoid conjugate (PRP-T), and (3) Haemophilus influenzae type b conjugate vaccine 4 Three HPV vaccines are available for use: (1) quadrivalent vaccine (HPV4) and (2) 9-valent vaccine (HPV9) for the prevention of cervical, vaginal, and vulvar cancers (in females) and genital warts (i
Primary Immunization Substitute one dose of Tdap for Td in all adults Four doses administered every other day One dose Two doses 4–8 weeks apart in persons past their 13th birthday (see Table A–2 for childhood schedule) One dose 10 years to 10 days before travel One dose
Route of Administration Intramuscular Oral Intramuscular Subcutaneous Subcutaneous Subcutaneous 1 Dosages for the specific product, including variations for age, are best obtained from the manufacturer’s package insert.
Type of Agent Toxoids and inac- tivated bacterial components Live bacteria Bacterial polysaccharide Live virus Live virus Live virus (meningococcal protein conjugate) (PRP-OMP). vaccine (HPV2) for the prevention of cervical cancers in females.
TABLE A–1 Vaccine Tetanus, diph- theria, pertussis (Tdap) Typhoid, Ty21a oral Typhoid, Vi capsular polysaccharide Varicella Yellow fever Zoster 2 One dose unless otherwise indicated. toxoid as DPT).
1178