Page 13 - E-Science Prep3 2024-2025 t1 Unit2_Neat
P. 13
Science department
Prep.3
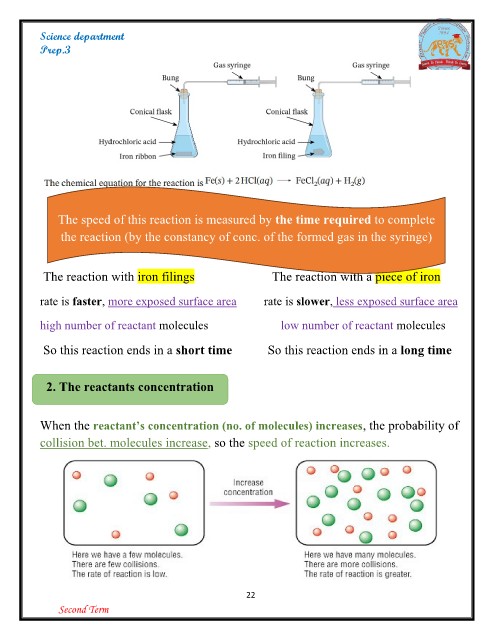
The speed of this reaction is measured by the time required to complete
the reaction (by the constancy of conc. of the formed gas in the syringe)
The reaction with iron filings The reaction with a piece of iron
rate is faster, more exposed surface area rate is slower, less exposed surface area
high number of reactant molecules
So this reaction ends in a short time low number of reactant molecules
So this reaction ends in a long time
2. The reactants concentration
When the reactant’s concentration (no. of molecules) increases, the probability of
collision bet. molecules increase, so the speed of reaction increases.
22
Second Term
Term