Page 8 - E-Science Prep3 2024-2025 t1 Unit2_Neat
P. 8
Science department
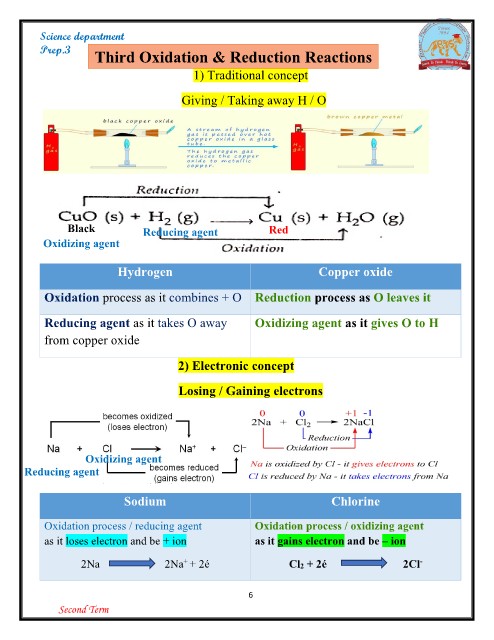
Prep.3 Third Oxidation & Reduction Reactions
1) Traditional concept
Giving / Taking away H / O
Black Reducing agent Red
Oxidizing agent
Hydrogen Copper oxide
Oxidation process as it combines + O Reduction process as O leaves it
Reducing agent as it takes O away Oxidizing agent as it gives O to H
from copper oxide
2) Electronic concept
Losing / Gaining electrons
Oxidizing agent Chlorine
Reducing agent
Oxidation process / oxidizing agent
Sodium as it gains electron and be – ion
Oxidation process / reducing agent Cl2 + 2é 2Cl-
as it loses electron and be + ion
6
2Na 2Na+ + 2é
Second Term
Term