Page 9 - E-Science Prep3 2024-2025 t1 Unit2_Neat
P. 9
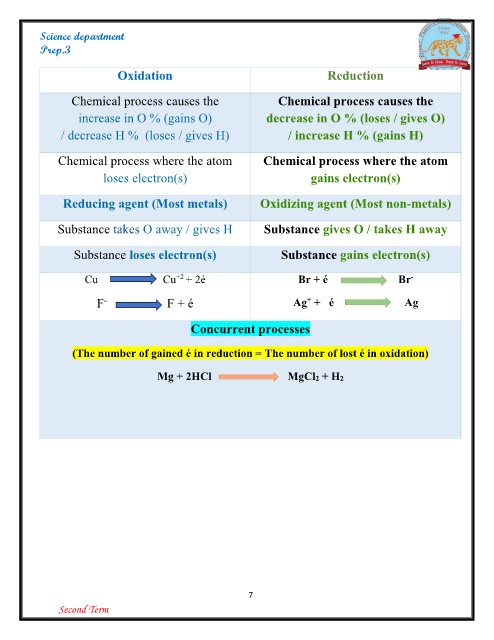
Science department
Prep.3
Oxidation Reduction
Chemical process causes the Chemical process causes the
increase in O % (gains O) decrease in O % (loses / gives O)
/ decrease H % (loses / gives H) / increase H % (gains H)
Chemical process where the atom Chemical process where the atom
loses electron(s) gains electron(s)
Reducing agent (Most metals) Oxidizing agent (Most non-metals)
Substance takes O away / gives H Substance gives O / takes H away
Substance loses electron(s) Substance gains electron(s)
Cu Cu+2 + 2é Br + é Br-
Ag+ + é Ag
F- F+é
Concurrent processes
(The number of gained é in reduction = The number of lost é in oxidation)
Mg + 2HCl MgCl2 + H2
7
Second Term
Term