Page 66 - Org 3 theoritical book 2024-25
P. 66
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
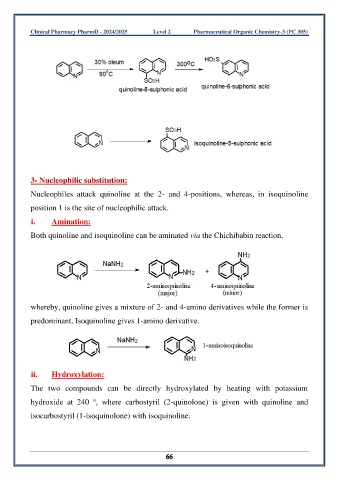
3- Nucleophilic substitution:
Nucleophiles attack quinoline at the 2- and 4-positions, whereas, in isoquinoline
position 1 is the site of nucleophilic attack.
i. Amination:
Both quinoline and isoquinoline can be aminated via the Chichibabin reaction,
whereby, quinoline gives a mixture of 2- and 4-amino derivatives while the former is
predominant. Isoquinoline gives 1-amino derivative.
ii. Hydroxylation:
The two compounds can be directly hydroxylated by heating with potassium
o
hydroxide at 240 , where carbostyril (2-quinolone) is given with quinoline and
isocarbostyril (1-isoquinolone) with isoquinoline.