Page 11 - che142 solutions ebook
P. 11
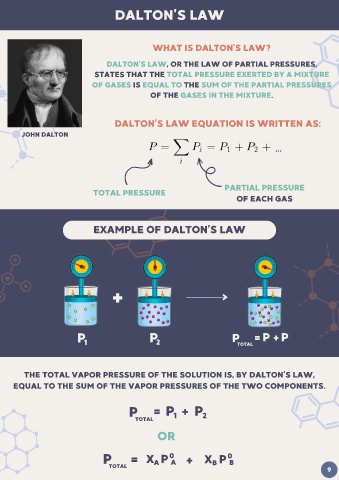
DALTON'S LAW
WHAT IS DALTON’S LAW?
DALTON’S LAW, OR THE LAW OF PARTIAL PRESSURES,
STATES THAT THE TOTAL PRESSURE EXERTED BY A MIXTURE
OF GASES IS EQUAL TO THE SUM OF THE PARTIAL PRESSURES
OF THE GASES IN THE MIXTURE.
DALTON’S LAW EQUATION IS WRITTEN AS:
JOHN DALTON
...
PARTIAL PRESSURE
TOTAL PRESSURE
OF EACH GAS
EXAMPLE OF DALTON’S LAW
+
P 1 P 2 P TOTAL = P + P 2
1
THE TOTAL VAPOR PRESSURE OF THE SOLUTION IS, BY DALTON’S LAW,
EQUAL TO THE SUM OF THE VAPOR PRESSURES OF THE TWO COMPONENTS.
P = P + P
TOTAL 1 2
OR
P = X P 0 X P 0
A +
TOTAL A B B
9